Natural cyclopeptide RA-V inhibits the NF-κB signaling pathway by targeting TAK1
- PMID: 29915207
- PMCID: PMC6006164
- DOI: 10.1038/s41419-018-0743-2
Natural cyclopeptide RA-V inhibits the NF-κB signaling pathway by targeting TAK1
Abstract
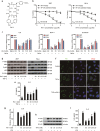
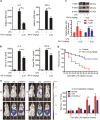
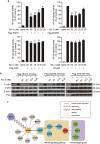
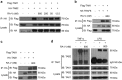
Rubiaceae-type cyclopeptides (RAs) are a type of plant cyclopeptides from the Rubia that have garnered significant attention owing to their unique bicyclic structures and amazing antitumour activities. Our recent work has shown that RAs suppress inflammation and angiogenesis and induce apoptosis. However, the underlying mechanism and targets remained unknown. Nuclear factor κB (NF-κB) signaling pathway plays a critical role in these biological processes, prompting us to investigate whether and how RAs affect this pathway. By screening compound libraries using NF-κB-dependent luciferase reporter, we observed that RA-V is the best NF-κB inhibitor. Further experiments demonstrated that RA-V interrupted the TAK1-TAB2 interaction and targeted TAK1 in this pathway. Moreover, RA-V prevented endotoxin shock and inhibited NF-κB activation and tumor growth in vivo. These findings clarify the mechanism of RA-V on NF-κB pathway and might account for the majority of known bioactivities of RA-V, which will help RA-V develop as new antiinflammatory and antitumour therapies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Phosphoinositide-dependent kinase-1 inhibits TRAF6 ubiquitination by interrupting the formation of TAK1-TAB2 complex in TLR4 signaling.Cell Signal. 2015 Dec;27(12):2524-33. doi: 10.1016/j.cellsig.2015.09.018. Epub 2015 Sep 30. Cell Signal. 2015. PMID: 26432169
-
Reciprocal inhibition between the transforming growth factor-β-activated kinase 1 (TAK1) and apoptosis signal-regulating kinase 1 (ASK1) mitogen-activated protein kinase kinase kinases and its suppression by TAK1-binding protein 2 (TAB2), an adapter protein for TAK1.J Biol Chem. 2012 Jan 27;287(5):3381-91. doi: 10.1074/jbc.M111.317875. Epub 2011 Dec 13. J Biol Chem. 2012. PMID: 22167179 Free PMC article.
-
Rubipodanin B, a New Cytotoxic Cyclopeptide from Rubia podantha.Chem Biodivers. 2019 Jan;16(1):e1800438. doi: 10.1002/cbdv.201800438. Epub 2018 Dec 13. Chem Biodivers. 2019. PMID: 30334345
-
TRAF6 and TAK1 Contribute to SAMHD1-Mediated Negative Regulation of NF-κB Signaling.J Virol. 2021 Jan 13;95(3):e01970-20. doi: 10.1128/JVI.01970-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177202 Free PMC article.
-
X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1).J Biol Chem. 2005 Nov 18;280(46):38599-608. doi: 10.1074/jbc.M505671200. Epub 2005 Sep 12. J Biol Chem. 2005. PMID: 16157589
Cited by
-
Redox Dual-Responsive and O2‑Evolving Theranostic Nanosystem for Highly Selective Chemotherapy against Hypoxic Tumors.Theranostics. 2019 Jan 1;9(1):90-103. doi: 10.7150/thno.30259. eCollection 2019. Theranostics. 2019. PMID: 30662556 Free PMC article.
-
Pharmacological Mechanisms of Shangke Huangshui against Skin and Soft Tissue Infection.Evid Based Complement Alternat Med. 2022 Feb 16;2022:9312611. doi: 10.1155/2022/9312611. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35222679 Free PMC article.
-
Cyclopeptide RA-V Inhibits Organ Enlargement and Tumorigenesis Induced by YAP Activation.Cancers (Basel). 2018 Nov 16;10(11):449. doi: 10.3390/cancers10110449. Cancers (Basel). 2018. PMID: 30453531 Free PMC article.
-
Natural Naphthohydroquinone Dimer Rubioncolin C Exerts Anti-Tumor Activity by Inducing Apoptotic and Autophagic Cell Death and Inhibiting the NF-κB and Akt/mTOR/P70S6K Pathway in Human Cancer Cells.Cells. 2019 Dec 7;8(12):1593. doi: 10.3390/cells8121593. Cells. 2019. PMID: 31817918 Free PMC article.
-
Anti-Apoptotic Role of Sanhuang Xiexin Decoction and Anisodamine in Endotoxemia.Front Pharmacol. 2021 Apr 21;12:531325. doi: 10.3389/fphar.2021.531325. eCollection 2021. Front Pharmacol. 2021. PMID: 33967742 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

