Liver X receptors regulate hepatic F4/80 + CD11b+ Kupffer cells/macrophages and innate immune responses in mice
- PMID: 29915246
- PMCID: PMC6006359
- DOI: 10.1038/s41598-018-27615-7
Liver X receptors regulate hepatic F4/80 + CD11b+ Kupffer cells/macrophages and innate immune responses in mice
Abstract
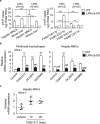
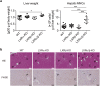
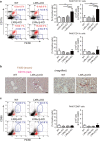
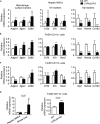
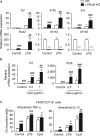
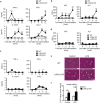
The liver X receptors (LXRs), LXRα and LXRβ, are nuclear receptors that regulate lipid homeostasis. LXRs also regulate inflammatory responses in cultured macrophages. However, the role of LXRs in hepatic immune cells remains poorly characterized. We investigated the role of LXRs in regulation of inflammatory responses of hepatic mononuclear cells (MNCs) in mice. Both LXRα and LXRβ were expressed in mouse hepatic MNCs and F4/80+ Kupffer cells/macrophages. LXRα/β-knockout (KO) mice had an increased number of hepatic MNCs and elevated expression of macrophage surface markers and inflammatory cytokines compared to wild-type (WT) mice. Among MNCs, F4/80+CD11b+ cells, not F4/80+CD11b- or F4/80+CD68+ cells, were increased in LXRα/β-KO mice more than WT mice. Isolated hepatic MNCs and F4/80+CD11b+ cells of LXRα/β-KO mice showed enhanced production of inflammatory cytokines after stimulation by lipopolysaccharide or CpG-DNA compared to WT cells, and LXR ligand treatment suppressed lipopolysaccharide-induced cytokine expression in hepatic MNCs. Lipopolysaccharide administration also stimulated inflammatory cytokine production in LXRα/β-KO mice more effectively than WT mice. Thus, LXR deletion enhances recruitment of F4/80+CD11b+ Kupffer cells/macrophages and acute immune responses in the liver. LXRs regulate the Kupffer cell/macrophage population and innate immune and inflammatory responses in mouse liver.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Liver X Receptors Regulate Cholesterol Metabolism and Immunity in Hepatic Nonparenchymal Cells.Int J Mol Sci. 2019 Oct 11;20(20):5045. doi: 10.3390/ijms20205045. Int J Mol Sci. 2019. PMID: 31614590 Free PMC article. Review.
-
Dysregulation of Kupffer Cells/Macrophages and Natural Killer T Cells in Steatohepatitis in LXRα Knockout Male Mice.Endocrinology. 2018 Mar 1;159(3):1419-1432. doi: 10.1210/en.2017-03141. Endocrinology. 2018. PMID: 29409022
-
Contrasting functional responses of resident Kupffer cells and recruited liver macrophages to irradiation and liver X receptor stimulation.PLoS One. 2021 Jul 23;16(7):e0254886. doi: 10.1371/journal.pone.0254886. eCollection 2021. PLoS One. 2021. PMID: 34297734 Free PMC article.
-
Involvement of the TNF and FasL produced by CD11b Kupffer cells/macrophages in CCl4-induced acute hepatic injury.PLoS One. 2014 Mar 25;9(3):e92515. doi: 10.1371/journal.pone.0092515. eCollection 2014. PLoS One. 2014. PMID: 24667392 Free PMC article.
-
Liver X receptors as regulators of macrophage inflammatory and metabolic pathways.Biochim Biophys Acta. 2011 Aug;1812(8):982-94. doi: 10.1016/j.bbadis.2010.12.015. Epub 2010 Dec 28. Biochim Biophys Acta. 2011. PMID: 21193033 Review.
Cited by
-
Are Liver Pericytes Just Precursors of Myofibroblasts in Hepatic Diseases? Insights from the Crosstalk between Perivascular and Inflammatory Cells in Liver Injury and Repair.Cells. 2020 Jan 11;9(1):188. doi: 10.3390/cells9010188. Cells. 2020. PMID: 31940814 Free PMC article. Review.
-
β-PIX cooperates with GIT1 to regulate endothelial nitric oxide synthase in sinusoidal endothelial cells.Am J Physiol Gastrointest Liver Physiol. 2022 Nov 1;323(5):G511-G522. doi: 10.1152/ajpgi.00034.2022. Epub 2022 Aug 31. Am J Physiol Gastrointest Liver Physiol. 2022. PMID: 36044673 Free PMC article.
-
Manjari Medika Grape Seed Extract Protects Methotrexate-Induced Hepatic Inflammation: Involvement of NF-κB/NLRP3 and Nrf2/HO-1 Signaling System.J Inflamm Res. 2023 Feb 7;16:467-492. doi: 10.2147/JIR.S338888. eCollection 2023. J Inflamm Res. 2023. PMID: 36785716 Free PMC article.
-
Dietary Cholesterol Metabolite Regulation of Tissue Immune Cell Development and Function.J Immunol. 2022 Aug 15;209(4):645-653. doi: 10.4049/jimmunol.2200273. J Immunol. 2022. PMID: 35961669 Free PMC article. Review.
-
Liver X Receptors Regulate Cholesterol Metabolism and Immunity in Hepatic Nonparenchymal Cells.Int J Mol Sci. 2019 Oct 11;20(20):5045. doi: 10.3390/ijms20205045. Int J Mol Sci. 2019. PMID: 31614590 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

