Platelet-derived growth factor receptor-α and -β promote cancer stem cell phenotypes in sarcomas
- PMID: 29915281
- PMCID: PMC6006341
- DOI: 10.1038/s41389-018-0059-1
Platelet-derived growth factor receptor-α and -β promote cancer stem cell phenotypes in sarcomas
Retraction in
-
Retraction Note: Platelet-derived growth factor receptor-α and -β promote cancer stem cell phenotypes in sarcomas.Oncogenesis. 2024 May 29;13(1):19. doi: 10.1038/s41389-024-00520-7. Oncogenesis. 2024. PMID: 38811545 Free PMC article. No abstract available.
Abstract
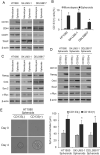
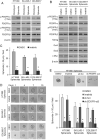
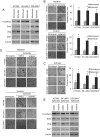
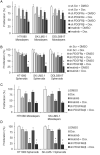
Sarcomas are malignant tumors derived from mesenchymal tissues and may harbor a subset of cells with cancer stem-like cell (CSC) properties. Platelet-derived growth factor receptors α and β (PDGFR-α/β) play an important role in the maintenance of mesenchymal stem cells. Here we examine the role of PDGFR-α/β in sarcoma CSCs. PDGFR-α/β activity and the effects of PDGFR-α/β inhibition were examined in 3 human sarcoma cell lines using in vitro assays and mouse xenograft models. In all three cell lines, PDGFR-α/β activity was significantly higher in cells grown as spheroids (to enrich for CSCs) and in cells sorted for CD133 expression (a marker of sarcoma CSCs). Self-renewal transcription factors Nanog, Oct4, and Slug and epithelial-to-mesenchymal transition (EMT) proteins Snail, Slug, and Zeb1 were also significantly higher in spheroids cells and CD133(+) cells. Spheroid cells and CD133(+) cells demonstrated 2.9- to 4.2-fold greater migration and invasion and resistance to doxorubicin chemotherapy. Inhibition of PDGFR-α/β in CSCs using shRNA or pharmacologic inhibitors reduced expression of certain self-renewal and EMT proteins, reduced spheroid formation by 74-82%, reduced migration and invasion by 73-80%, and reversed chemotherapy resistance. In mouse xenograft models, combining PDGFR-α/β inhibition (using shRNA or imatinib) with doxorubicin had a more-than-additive effect in blocking tumor growth, with enhanced apoptosis, especially in CD133(+) cells. These results indicate that PDGFR-α/β activity is upregulated in sarcoma CSCs and promote CSC phenotypes including migration, invasion, and chemotherapy resistance. Thus, the PDGFR-α/β pathway represents a new potential therapeutic target to reduce metastatic potential and increase chemosensitivity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Van Glabbeke M, et al. Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J. Clin. Oncol. 1999;17:150–157. doi: 10.1200/JCO.1999.17.1.150. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

