Low-affinity Nerve Growth Factor Receptor (CD271) Heterogeneous Expression in Adult and Fetal Mesenchymal Stromal Cells
- PMID: 29915318
- PMCID: PMC6006357
- DOI: 10.1038/s41598-018-27587-8
Low-affinity Nerve Growth Factor Receptor (CD271) Heterogeneous Expression in Adult and Fetal Mesenchymal Stromal Cells
Abstract
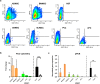
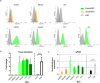
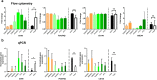
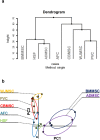
Human multipotent mesenchymal stromal cells (MSC) are isolated from a plethora of tissue sources for cell therapy purposes. In 2006, the International Society for Cellular Therapy (ISCT) published minimal guidelines to define MSC identity. Nevertheless, many independent studies demonstrated that cells meeting the ISCT criteria possessed heterogeneous phenotypes and functionalities, heavily influenced by culture conditions. In this study, human MSC derived from many adult (bone marrow and adipose tissue) or fetal (cord blood, Wharton's jelly, umbilical cord perivascular compartment and amniotic fluid) tissues were investigated. Their immunophenotype was analyzed to define consistent source-specific markers by extensive flow cytometry analysis and real-time qRT-PCR. CD271+ subpopulations were detected in adult MSC, whereas NG2 was significantly more expressed in fetal MSC but failed validation on independent samples coming from an external laboratory. The highest number of CD271+ adult MSC were detected soon after isolation in serum-based culture conditions. Furthermore, heterogeneous percentages of CD271 expression were found in platelet lysate-based or serum-free culture conditions. Finally, CD271+ adult MSC showed high clonogenic and osteogenic properties as compared to CD271- cells. To conclude, in this phenotype-function correlation study CD271+ subpopulation confers heterogeneity on adult MSC, confirming the need of more specific markers to address MSC properties.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Adipogenic potential in human mesenchymal stem cells strictly depends on adult or foetal tissue harvest.Int J Biochem Cell Biol. 2013 Nov;45(11):2456-66. doi: 10.1016/j.biocel.2013.07.024. Epub 2013 Aug 11. Int J Biochem Cell Biol. 2013. PMID: 23942228
-
High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources.Stem Cell Res Ther. 2018 Jan 16;9(1):10. doi: 10.1186/s13287-017-0755-3. Stem Cell Res Ther. 2018. PMID: 29338788 Free PMC article.
-
Large-scale extraction and characterization of CD271+ multipotential stromal cells from trabecular bone in health and osteoarthritis: implications for bone regeneration strategies based on uncultured or minimally cultured multipotential stromal cells.Arthritis Rheum. 2010 Jul;62(7):1944-54. doi: 10.1002/art.27451. Arthritis Rheum. 2010. PMID: 20222109
-
CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture.World J Stem Cells. 2015 Mar 26;7(2):470-6. doi: 10.4252/wjsc.v7.i2.470. World J Stem Cells. 2015. PMID: 25815130 Free PMC article. Review.
-
Markers of stemness in equine mesenchymal stem cells: a plea for uniformity.Theriogenology. 2011 May;75(8):1431-43. doi: 10.1016/j.theriogenology.2010.11.008. Epub 2010 Dec 31. Theriogenology. 2011. PMID: 21196039 Review.
Cited by
-
CD271+ stem cell treatment of patients with chronic stroke: : A retrospective case series report.Exp Ther Med. 2020 Sep;20(3):2055-2062. doi: 10.3892/etm.2020.8948. Epub 2020 Jun 26. Exp Ther Med. 2020. PMID: 32782517 Free PMC article.
-
Adipose stem cell niche reprograms the colorectal cancer stem cell metastatic machinery.Nat Commun. 2021 Aug 18;12(1):5006. doi: 10.1038/s41467-021-25333-9. Nat Commun. 2021. PMID: 34408135 Free PMC article.
-
A flow cytometric assay for the quantification of MSC lysis by peripheral blood mononucleated cells.Heliyon. 2021 Feb 1;7(2):e06036. doi: 10.1016/j.heliyon.2021.e06036. eCollection 2021 Feb. Heliyon. 2021. PMID: 33553772 Free PMC article.
-
FOXP1 circular RNA sustains mesenchymal stem cell identity via microRNA inhibition.Nucleic Acids Res. 2019 Jun 4;47(10):5325-5340. doi: 10.1093/nar/gkz199. Nucleic Acids Res. 2019. PMID: 30937446 Free PMC article.
-
Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application.NPJ Regen Med. 2021 Mar 19;6(1):14. doi: 10.1038/s41536-021-00122-6. NPJ Regen Med. 2021. PMID: 33741999 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

