Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis
- PMID: 29915579
- PMCID: PMC5994487
- DOI: 10.3389/fimmu.2018.01197
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis
Abstract
Background: Inflammasomes, which mediate maturation of interleukin-1β (IL-β) and interleukin-18 (IL-18) and lead to pyroptosis, have been linked to various autoimmune disorders. This study investigated whether they are involved in the pathogenesis of autoimmune thyroiditis (AIT).
Methods: We collected thyroid tissues from 50 patients with AIT and 50 sex- and age-matched controls. Serum levels of free T3, free T4, thyrotropin, thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TgAb) were measured by electrochemiluminescent immunoassays. Expression of several inflammasome components, the NOD-like receptor (NLR) family pyrin domain containing 1 (NLRP1), NLRP3, CARD-domain containing 4 (NLRC4), absent in melanoma 2 (AIM2), the apoptosis-associated speck-like protein that contains a caspase recruitment domain (ASC), caspase-1, IL-1β, and IL-18 was determined by real-time PCR and western blot. Immunohistochemistry was used to localize the expression of NLRP1, NLRP3, NLRC4, and AIM2. The Nthy-ori 3-1 thyroid cell line was stimulated with tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-17A, interleukin-6, and poly(dA:dT). The levels of IL-18 and IL-1β in the cell supernatant were measured by enzyme-linked immunosorbent assay, and lactate dehydrogenase was quantified by absorptiometry. ASC specks were examined by confocal immunofluorescence microscopic analysis. Cell death was examined by flow cytometry, and the N-terminal domain of gasdermin D was detected by western blot analysis.
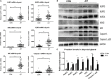
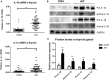
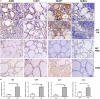
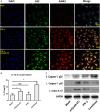
Results: Expression of NLRP1, NLRP3, NLRC4, AIM2, ASC, caspase-1, pro IL-1β, pro IL-18, mRNA, and protein was significantly increased in thyroid tissues from patients with AIT, and enhanced posttranslational maturation of caspase-1, IL-18 and IL-1β was also observed. Expression of NLRP1, NLRP3, NLRC4, and AIM2 was localized mainly in thyroid follicular cells adjacent to areas of lymphatic infiltration. The thyroid mRNA level of NLRP1 and ASC was correlated to the serum TPOAb and TgAb levels in the AIT group. TNF-α and IFN-γ had a priming effect on the expression of multiple inflammasome components in thyroid cells. IFN-γ was found to strengthen poly(dA:dT)-induced cell pyroptosis and bioactive IL-18 release.
Conclusion: Our work has demonstrated for the first time that multiple inflammasomes are associated with AIT pathogenesis. The identified NLRP3, NLRP1, NLRC4, AIM2 inflammasomes and their downstream cytokines may represent potential therapeutic targets and biomarkers of AIT.
Keywords: absent in melanoma 2; autoimmune thyroiditis; inflammasome; interleukin-18 (IL-18); pyroptosis.
Figures



Similar articles
-
AIM2 and NLRC4-driven inflammasome activation in adult-onset Still's disease and the preliminary therapeutic effect exploration of carboxyamidotriazole.Clin Rheumatol. 2023 Jun;42(6):1635-1643. doi: 10.1007/s10067-022-06443-1. Epub 2022 Nov 24. Clin Rheumatol. 2023. PMID: 36418508
-
HIV replication is associated to inflammasomes activation, IL-1β, IL-18 and caspase-1 expression in GALT and peripheral blood.PLoS One. 2018 Apr 19;13(4):e0192845. doi: 10.1371/journal.pone.0192845. eCollection 2018. PLoS One. 2018. PMID: 29672590 Free PMC article.
-
NLRC4, ASC and Caspase-1 Are Inflammasome Components that Are Mediated by P2Y2R Activation in Breast Cancer Cells.Int J Mol Sci. 2020 May 8;21(9):3337. doi: 10.3390/ijms21093337. Int J Mol Sci. 2020. PMID: 32397236 Free PMC article.
-
Recent advances in inflammasome biology.Curr Opin Immunol. 2018 Feb;50:32-38. doi: 10.1016/j.coi.2017.10.011. Epub 2017 Nov 10. Curr Opin Immunol. 2018. PMID: 29128729 Free PMC article. Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
Magnetic resonance T1-mapping quantitatively assesses the severity of thyroid destruction in patients with autoimmune thyroiditis.Front Endocrinol (Lausanne). 2022 Nov 1;13:1028588. doi: 10.3389/fendo.2022.1028588. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36387897 Free PMC article.
-
Expression profile of inflammasome genes in individuals with Down syndrome.Genet Mol Biol. 2024 Sep 9;47(4):e20230339. doi: 10.1590/1678-4685-GMB-2023-0339. eCollection 2024. Genet Mol Biol. 2024. PMID: 39264098 Free PMC article.
-
Emerging insights on the role of gasdermins in infection and inflammatory diseases.Clin Transl Immunology. 2020 Oct 4;9(10):e1186. doi: 10.1002/cti2.1186. eCollection 2020. Clin Transl Immunology. 2020. PMID: 33033617 Free PMC article. Review.
-
Excessive Iodine Promotes Pyroptosis of Thyroid Follicular Epithelial Cells in Hashimoto's Thyroiditis Through the ROS-NF-κB-NLRP3 Pathway.Front Endocrinol (Lausanne). 2019 Nov 20;10:778. doi: 10.3389/fendo.2019.00778. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31824415 Free PMC article.
-
Association Between Gut Microbiota and Autoimmune Thyroid Disease: A Systematic Review and Meta-Analysis.Front Endocrinol (Lausanne). 2021 Nov 17;12:774362. doi: 10.3389/fendo.2021.774362. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867823 Free PMC article.
References
-
- Dong YH, Fu DG. Autoimmune thyroid disease: mechanism, genetics and current knowledge. Eur Rev Med Pharmacol Sci (2014) 18(23):3611–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

