Immunoglobulin G Fragment Crystallizable Glycosylation After Hematopoietic Stem Cell Transplantation Is Dissimilar to Donor Profiles
- PMID: 29915589
- PMCID: PMC5994695
- DOI: 10.3389/fimmu.2018.01238
Immunoglobulin G Fragment Crystallizable Glycosylation After Hematopoietic Stem Cell Transplantation Is Dissimilar to Donor Profiles
Abstract
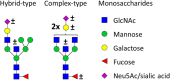
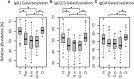
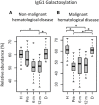
Immunoglobulin G (IgG) fragment crystallizable (Fc) N-glycosylation has a large influence on the affinity of the antibody for binding to Fcγ-receptors (FcγRs) and C1q protein, thereby influencing immune effector functions. IgG Fc glycosylation is known to be partly regulated by genetics and partly by stimuli in the microenvironment of the B cell. Following allogeneic hematopoietic stem cell transplantation (HSCT), and in the presence of (almost) complete donor chimerism, IgG is expected to be produced by, and glycosylated in, B cells of donor origin. We investigated to what extent IgG glycosylation in patients after transplantation is determined by factors of the donor (genetics) or the recipient (environment). Using an IgG subclass-specific liquid chromatography-mass spectrometry method, we analyzed the plasma/serum IgG Fc glycosylation profiles of 34 pediatric patients pre-HSCT and at 6 and 12 months post-HSCT and compared these to the profiles of their donors and age-matched healthy controls. Patients treated for hematological malignancies as well as for non-malignant hematological diseases showed after transplantation a lower Fc galactosylation than their donors. Especially for the patients treated for leukemia, the post-HSCT Fc glycosylation profiles were more similar to the pre-HSCT recipient profiles than to profiles of the donors. Pre-HSCT, the leukemia patient group showed as distinctive feature a decrease in sialylation and in hybrid-type glycans as compared to healthy controls, which both normalized after transplantation. Our data suggest that IgG Fc glycosylation in children after HSCT does not directly mimic the donor profile, but is rather determined by persisting environmental factors of the host.
Keywords: N-glycan; fragment crystallizable glycosylation; hematopoietic stem cell transplantation; immune reconstitution; immunoglobulin G.
Figures


Similar articles
-
Differences in IgG Fc Glycosylation Are Associated with Outcome of Pediatric Meningococcal Sepsis.mBio. 2018 Jun 19;9(3):e00546-18. doi: 10.1128/mBio.00546-18. mBio. 2018. PMID: 29921663 Free PMC article.
-
A functional spleen contributes to afucosylated IgG in humans.Sci Rep. 2021 Dec 15;11(1):24045. doi: 10.1038/s41598-021-03196-w. Sci Rep. 2021. PMID: 34911982 Free PMC article.
-
Changes in Healthy Human IgG Fc-Glycosylation after Birth and during Early Childhood.J Proteome Res. 2016 Jun 3;15(6):1853-61. doi: 10.1021/acs.jproteome.6b00038. Epub 2016 May 19. J Proteome Res. 2016. PMID: 27161864
-
Alternative Donor Graft Sources for Adults with Hematologic Malignancies: A Donor for All Patients in 2017!Oncologist. 2017 Sep;22(9):1125-1134. doi: 10.1634/theoncologist.2017-0009. Epub 2017 May 25. Oncologist. 2017. PMID: 28546462 Free PMC article. Review.
-
IgG N-glycans.Adv Clin Chem. 2021;105:1-47. doi: 10.1016/bs.acc.2021.02.001. Epub 2021 Mar 18. Adv Clin Chem. 2021. PMID: 34809825 Review.
Cited by
-
Set Up for Failure: Pre-Existing Autoantibodies in Lung Transplant.Front Immunol. 2021 Aug 11;12:711102. doi: 10.3389/fimmu.2021.711102. eCollection 2021. Front Immunol. 2021. PMID: 34456920 Free PMC article. Review.
-
Analysis of monoclonal immunoglobulins from bone marrow plasma cells using immunopurification and LC-MS.J Mass Spectrom Adv Clin Lab. 2023 Apr 15;28:133-141. doi: 10.1016/j.jmsacl.2023.04.001. eCollection 2023 Apr. J Mass Spectrom Adv Clin Lab. 2023. PMID: 37138663 Free PMC article.
-
Polyfunctional antibodies: a path towards precision vaccines for vulnerable populations.Front Immunol. 2023 Jun 27;14:1183727. doi: 10.3389/fimmu.2023.1183727. eCollection 2023. Front Immunol. 2023. PMID: 37600816 Free PMC article. Review.
-
Site-Specific N-Glycan Characterization of Grass Carp Serum IgM.Front Immunol. 2018 Nov 14;9:2645. doi: 10.3389/fimmu.2018.02645. eCollection 2018. Front Immunol. 2018. PMID: 30487799 Free PMC article.
-
Immunoglobulin G Glycosylation Changes in Aging and Other Inflammatory Conditions.Exp Suppl. 2021;112:303-340. doi: 10.1007/978-3-030-76912-3_10. Exp Suppl. 2021. PMID: 34687015
References
-
- Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A (2011) 108(31):12669–74.10.1073/pnas.1108455108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

