Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves
- PMID: 29915612
- PMCID: PMC5994434
- DOI: 10.3389/fpls.2018.00756
Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves
Abstract
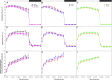
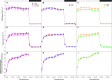
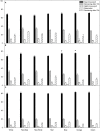
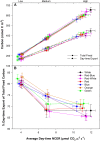
Translocation of assimilates is a fundamental process involving carbon and water balance affecting source/sink relationships. Diurnal patterns of CO2 exchange, translocation (carbon export), and transpiration of an intact tomato source leaf were determined during 14CO2 steady-state labeling under different wavelengths at three pre-set photosynthetic rates. Daily patterns showed that photosynthesis and export were supported by all wavelengths of light tested including orange and green. Export in the light, under all wavelengths was always higher than that at night. Export in the light varied from 65-83% of the total daily carbon fixed, depending on light intensity. Photosynthesis and export were highly correlated under all wavelengths (r = 0.90-0.96). Export as a percentage of photosynthesis (relative export) decreased as photosynthesis increased by increasing light intensity under all wavelengths. These data indicate an upper limit for export under all spectral conditions. Interestingly, only at the medium photosynthetic rate, relative export under the blue and the orange light-emitting diodes (LEDs) were higher than under white and red-white LEDs. Stomatal conductance, transpiration rates, and water-use-efficiency showed similar daily patterns under all wavelengths. Illuminating tomato leaves with different spectral quality resulted in similar carbon export rates, but stomatal conductance and transpiration rates varied due to wavelength specific control of stomatal function. Thus, we caution that the link between transpiration and C-export may be more complex than previously thought. In summary, these data indicate that orange and green LEDs, not simply the traditionally used red and blue LEDs, should be considered and tested when designing lighting systems for optimizing source leaf strength during plant production in controlled environment systems. In addition, knowledge related to the interplay between water and C-movement within a plant and how they are affected by environmental stimuli, is needed to develop a better understanding of source/sink relationships.
Keywords: carbon export; light quality; light-emitting diode (LED); photosynthesis; tomato; translocation; transpiration; water-use-efficiency (WUE).
Figures



Similar articles
-
Effect of elevated CO2 and spectral quality on whole plant gas exchange patterns in tomatoes.PLoS One. 2018 Oct 18;13(10):e0205861. doi: 10.1371/journal.pone.0205861. eCollection 2018. PLoS One. 2018. PMID: 30335803 Free PMC article.
-
The Effect of Spectral Quality on Daily Patterns of Gas Exchange, Biomass Gain, and Water-Use-Efficiency in Tomatoes and Lisianthus: An Assessment of Whole Plant Measurements.Front Plant Sci. 2017 Jun 20;8:1076. doi: 10.3389/fpls.2017.01076. eCollection 2017. Front Plant Sci. 2017. PMID: 28676816 Free PMC article.
-
Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.).Int J Mol Sci. 2014 Mar 17;15(3):4657-70. doi: 10.3390/ijms15034657. Int J Mol Sci. 2014. PMID: 24642884 Free PMC article.
-
Alternating Red and Blue Light-Emitting Diodes Allows for Injury-Free Tomato Production With Continuous Lighting.Front Plant Sci. 2019 Sep 13;10:1114. doi: 10.3389/fpls.2019.01114. eCollection 2019. Front Plant Sci. 2019. PMID: 31572419 Free PMC article.
-
Mesophyll conductance in land surface models: effects on photosynthesis and transpiration.Plant J. 2020 Feb;101(4):858-873. doi: 10.1111/tpj.14587. Epub 2019 Dec 10. Plant J. 2020. PMID: 31659806 Review.
Cited by
-
Growth, phytochemical, and phytohormonal responses of basil to different light durations and intensities under constant daily light integral.BMC Plant Biol. 2024 Oct 9;24(1):935. doi: 10.1186/s12870-024-05637-w. BMC Plant Biol. 2024. PMID: 39379825 Free PMC article.
-
The growth, nutrient uptake and fruit quality in four strawberry cultivars under different Spectra of LED supplemental light.BMC Plant Biol. 2024 Mar 8;24(1):179. doi: 10.1186/s12870-024-04880-5. BMC Plant Biol. 2024. PMID: 38454341 Free PMC article.
-
Intra-canopy LED lighting outperformed top LED lighting in improving tomato yield and expression of the genes responsible for lycopene, phytoene and vitamin C synthesis.Sci Rep. 2024 Aug 16;14(1):19043. doi: 10.1038/s41598-024-69210-z. Sci Rep. 2024. PMID: 39152138 Free PMC article.
-
A Noninvasive Gas Exchange Method to Test and Model Photosynthetic Proficiency and Growth Rates of In Vitro Plant Cultures: Preliminary Implication for Cannabis sativa L.Biology (Basel). 2022 May 10;11(5):729. doi: 10.3390/biology11050729. Biology (Basel). 2022. PMID: 35625457 Free PMC article.
-
Non-invasive 11C-Imaging Revealed the Spatiotemporal Variability in the Translocation of Photosynthates Into Strawberry Fruits in Response to Increasing Daylight Integrals at Leaf Surface.Front Plant Sci. 2021 Jul 14;12:688887. doi: 10.3389/fpls.2021.688887. eCollection 2021. Front Plant Sci. 2021. PMID: 34335656 Free PMC article.
References
-
- Bertram L., Karlsen P. (1994). Patterns in stem elongation rate in chrysanthemum and tomato plants in relation to irradiance and day/night temperature. Sci. Hortic. 58 139–150. 10.1016/0304-4238(94)90134-1 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

