Megalin mediates plasma membrane to mitochondria cross-talk and regulates mitochondrial metabolism
- PMID: 29916093
- PMCID: PMC11105752
- DOI: 10.1007/s00018-018-2847-3
Megalin mediates plasma membrane to mitochondria cross-talk and regulates mitochondrial metabolism
Abstract
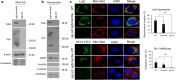
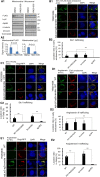
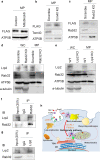
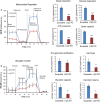
Mitochondrial intracrines are extracellular signaling proteins, targeted to the mitochondria. The pathway for mitochondrial targeting of mitochondrial intracrines and actions in the mitochondria remains unknown. Megalin/LRP2 mediates the uptake of vitamins and proteins, and is critical for clearance of amyloid-β protein from the brain. Megalin mutations underlie the pathogenesis of Donnai-Barrow and Lowe syndromes, characterized by brain defects and kidney dysfunction; megalin was not previously known to reside in the mitochondria. Here, we show megalin is present in the mitochondria and associates with mitochondrial anti-oxidant proteins SIRT3 and stanniocalcin-1 (STC1). Megalin shuttles extracellularly-applied STC1, angiotensin II and TGF-β to the mitochondria through the retrograde early endosome-to-Golgi transport pathway and Rab32. Megalin knockout in cultured cells impairs glycolytic and respiratory capacities. Thus, megalin is critical for mitochondrial biology; mitochondrial intracrine signaling is a continuum of the retrograde early endosome-to-Golgi-Rab32 pathway and defects in this pathway may underlie disease processes in many systems.
Keywords: ApoE; OCRL1; PIKfyve; Proteinuria; Sonic hedgehog; Vitamin D.
Conflict of interest statement
No financial interests to disclose.
Figures





References
-
- Birn H, Verroust PJ, Nexo E, Hager H, Jacobsen C, Christensen EI, Moestrup SK. Characterization of an epithelial approximately 460-kDa protein that facilitates endocytosis of intrinsic factor-vitamin B12 and binds receptor-associated protein. J Biol Chem. 1997;272:26497–26504. doi: 10.1074/jbc.272.42.26497. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

