The requirement for calcification differs between ecologically important coccolithophore species
- PMID: 29916209
- PMCID: PMC6175242
- DOI: 10.1111/nph.15272
The requirement for calcification differs between ecologically important coccolithophore species
Abstract
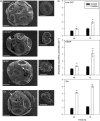
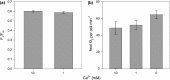
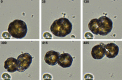
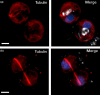
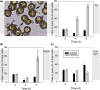
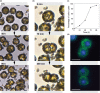
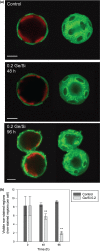
Coccolithophores are globally distributed unicellular marine algae that are characterized by their covering of calcite coccoliths. Calcification by coccolithophores contributes significantly to global biogeochemical cycles. However, the physiological requirement for calcification remains poorly understood as non-calcifying strains of some commonly used model species, such as Emiliania huxleyi, grow normally in laboratory culture. To determine whether the requirement for calcification differs between coccolithophore species, we utilized multiple independent methodologies to disrupt calcification in two important species of coccolithophore: E. huxleyi and Coccolithus braarudii. We investigated their physiological response and used time-lapse imaging to visualize the processes of calcification and cell division in individual cells. Disruption of calcification resulted in major growth defects in C. braarudii, but not in E. huxleyi. We found no evidence that calcification supports photosynthesis in C. braarudii, but showed that an inability to maintain an intact coccosphere results in cell cycle arrest. We found that C. braarudii is very different from E. huxleyi as it exhibits an obligate requirement for calcification. The identification of a growth defect in C. braarudii resulting from disruption of the coccosphere may be important in considering their response to future changes in ocean carbonate chemistry.
Keywords: Coccolithus braarudii; Emiliania huxleyi; calcification; coccolithophore; phytoplankton.
© 2018 The Authors. New Phytologist © 2018 New Phytologist Trust.
Figures


Comment in
-
Looking away from the streetlight - new insights into marine calcification.New Phytol. 2018 Oct;220(1):5-7. doi: 10.1111/nph.15409. New Phytol. 2018. PMID: 30156024 No abstract available.
References
-
- Abràmoff MD, Magalhães PJ, Ram SJ. 2004. Image processing with ImageJ. Biophotonics International 11: 36–42.
-
- Asahina M, Okazaki M. 2004. Inhibition of crystal growth in coccolith formation of Pleurochrysis carterae by a potent scale inhibitor, (1‐hydroxyethylidene) bisphosphonic acid (HEBP). Thalassas 20: 51–58.
-
- Azam F, Volcani B. 1981. Germanium–silicon interactions in biological systems In: Simpson TL, Volcani BE, eds. Silicon and siliceous structures in biological systems. New York, NY, USA: Springer, 43–67.
-
- Bach LT, Mackinder LC, Schulz KG, Wheeler G, Schroeder DC, Brownlee C, Riebesell U. 2013. Dissecting the impact of CO2 and pH on the mechanisms of photosynthesis and calcification in the coccolithophore. Emiliania huxleyi . New Phytologist 199: 121–134. - PubMed
-
- Bach LT, Riebesell U, Gutowska MA, Federwisch L, Schulz KG. 2015. A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Progress in Oceanography 135: 125–138.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

