Macroporous Dual-compartment Hydrogels for Minimally Invasive Transplantation of Primary Human Hepatocytes
- PMID: 29916986
- PMCID: PMC6102079
- DOI: 10.1097/TP.0000000000002330
Macroporous Dual-compartment Hydrogels for Minimally Invasive Transplantation of Primary Human Hepatocytes
Abstract
Background: Given the shortage of available organs for whole or partial liver transplantation, hepatocyte cell transplantation has long been considered a potential strategy to treat patients suffering from various liver diseases. Some of the earliest approaches that attempted to deliver hepatocytes via portal vein or spleen achieved little success due to poor engraftment. More recent efforts include transplantation of cell sheets or thin hepatocyte-laden synthetic hydrogels. However, these implants must remain sufficiently thin to ensure that nutrients can diffuse into the implant.
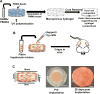
Methods: To circumvent these limitations, we investigated the use of a vascularizable dual-compartment hydrogel system for minimally invasive transplantation of primary hepatocytes. The dual-compartment system features a macroporous outer polyethylene glycol diacrylate/hyaluronic acid methacrylate hydrogel compartment for seeding supportive cells and facilitating host cell infiltration and vascularization and a hollow inner core to house the primary human hepatocytes.
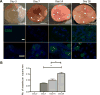
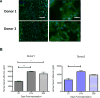
Results: We show that the subcutaneous implantation of these cell-loaded devices in NOD/SCID mice facilitated vascular formation while supporting viability of the transplanted cells. Furthermore, the presence of human serum albumin in peripheral blood and the immunostaining of excised implants indicated that the hepatocytes maintained function in vivo for at least 1 month, the longest assayed time point.
Conclusions: Cell transplantation devices that assist the anastomosis of grafts with the host can be potentially used as a minimally invasive ectopic liver accessory to augment liver-specific functions as well as potentially treat various pathologies associated with compromised functions of liver, such as hemophilia B or alpha-1 antitrypsin deficiency.
Figures



Comment in
-
Minimally Invasive Transplantation of Primary Human Hepatocyte Inserts that Facilitate Vascularization.Transplantation. 2018 Sep;102(9):1413-1414. doi: 10.1097/TP.0000000000002331. Transplantation. 2018. PMID: 29979347 No abstract available.
Similar articles
-
Human hepatocytes loaded in 3D bioprinting generate mini-liver.Hepatobiliary Pancreat Dis Int. 2016 Oct;15(5):512-518. doi: 10.1016/s1499-3872(16)60119-4. Hepatobiliary Pancreat Dis Int. 2016. PMID: 27733321
-
Cellularized biosynthetic microhydrogel polymers for intravascular liver tissue regeneration therapy.Tissue Eng Part A. 2014 Nov;20(21-22):2850-9. doi: 10.1089/ten.TEA.2013.0494. Epub 2014 Aug 19. Tissue Eng Part A. 2014. PMID: 24797901 Free PMC article.
-
Primary human hepatocytes on biodegradable poly(l-lactic acid) matrices: a promising model for improving transplantation efficiency with tissue engineering.Liver Transpl. 2011 Feb;17(2):104-14. doi: 10.1002/lt.22200. Liver Transpl. 2011. PMID: 21280182
-
[Potential of cell transplantation as an alternative to liver transplantation].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011 Apr;28(2):405-9. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011. PMID: 21604511 Review. Chinese.
-
Macroporous Hydrogel Scaffolds for Three-Dimensional Cell Culture and Tissue Engineering.Tissue Eng Part B Rev. 2017 Oct;23(5):451-461. doi: 10.1089/ten.TEB.2016.0465. Epub 2017 Feb 3. Tissue Eng Part B Rev. 2017. PMID: 28067115 Review.
Cited by
-
Molecularly Tailored Interface for Long-Term Xenogeneic Cell Transplantation.Adv Funct Mater. 2022 Jan 19;32(4):2108221. doi: 10.1002/adfm.202108221. Epub 2021 Oct 7. Adv Funct Mater. 2022. PMID: 37920452 Free PMC article.
References
-
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. National Vital Statistics Report. 14 Vol. 57. Hyattsville, MD: Centers for Disease Control and Prevention; 2009. - PubMed
-
- Schramm C, Bubenheim M, Adam R, et al. Primary liver transplantation for autoimmune hepatitis: a comparative analysis of the European Liver Transplant Registry. Liver Transpl. 2010;16(4):461–469. - PubMed
-
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. - PubMed
-
- Ambrosino G, Varotto S, Strom SC, et al. Isolated Hepatocyte Transplantation for Crigler-Najjar Syndrome Type 1. Cell Transplant. 2005;14(2–3):151–157. - PubMed
-
- Fox IJ, Chowdhury JR, Kaufman SS, et al. Treatment of the Crigler–Najjar Syndrome Type I with Hepatocyte Transplantation. N Engl J Med. 1998;338(20):1422–1427. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

