Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease
- PMID: 29917092
- PMCID: PMC6367993
- DOI: 10.1093/nutrit/nuy022
Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease
Abstract
Context: Treatment options for nonalcoholic fatty liver disease (NAFLD) are needed.
Objective: The aim of this review was to systematically assess the effects of omega-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs), particularly eicosapentaenoic acid and docosahexaenoic acid, on liver-related and metabolic outcomes in adult and pediatric patients with NAFLD.
Data sources: The online information service ProQuest Dialog was used to search 8 literature databases.
Study selection: Controlled intervention studies in which the independent effects of n-3 LC-PUFAs could be isolated were eligible for inclusion.
Data extraction: The 18 unique studies that met the criteria for inclusion were divided into 2 sets, and data transcriptions and study quality assessments were conducted in duplicate. Each effect size was expressed as the weighted mean difference and 95%CI, using a random-effects model and the inverse of the variance as a weighting factor.
Results: Based on the meta-analyses, supplementation with n-3 LC-PUFAs resulted in statistically significant improvements in 6 of 13 metabolic risk factors, in levels of 2 of 3 liver enzymes, in liver fat content (assessed via magnetic resonance imaging/spectroscopy), and in steatosis score (assessed via ultrasonography). Histological measures of disease [which were assessed only in patients with nonalcoholic steatohepatitis (NASH)] were unaffected by n-3 LC-PUFA supplementation.
Conclusions: Omega-3 LC-PUFAs are useful in the dietary management of patients with NAFLD. Additional trials are needed to better understand the effects of n-3 LC-PUFAs on histological outcomes in patients with NASH.
Systematic review registration: PROSPERO CRD42017055951.
Figures

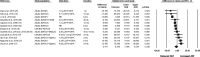


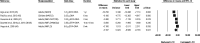

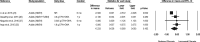
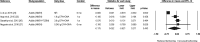
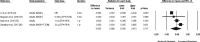
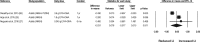

References
-
- Chalasani N, Younossi Z, Lavine JE et al. , . The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–826. - PubMed
-
- Petäjä EM, Yki-Järvinen H.. Definitions of normal liver fat and the association of insulin sensitivity with acquired and genetic NAFLD—a systematic review. Int J Mol Sci. 2016;17:633. doi:10.3390/ijms17050633 [16pp]. doi: 10.3390/ijms1705063. - DOI - PMC - PubMed
-
- Neuschwander-Tetri BA. Non-alcoholic fatty liver disease. BMC Med. 2017;15:45. doi:10.1186/s12916-017-0806-8 [6pp]. - DOI - PMC - PubMed
-
- Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(suppl 1):81–84. - PubMed
-
- Marchesini G, Petta S, Dalle Grave R.. Diet, weight loss, and liver health in nonalcoholic fatty liver disease: pathophysiology, evidence, and practice. Hepatology. 2016;63:2032–2043. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

