Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice
- PMID: 29917235
- PMCID: PMC6117588
- DOI: 10.1113/JP276079
Classification of GABAergic neuron subtypes from the globus pallidus using wild-type and transgenic mice
Abstract
Key points: Classifying different subtypes of neurons in deep brain structures is a challenge and is crucial to better understand brain function. Understanding the diversity of neurons in the globus pallidus (GP), a brain region positioned to influence afferent and efferent information processing within basal ganglia, could help to explain a variety of brain functions. We present a classification of neurons from the GP using electrophysiological data from wild-type mice and confirmation using transgenic mice. This work will help researchers to identify specific neuronal subsets in the GP of wild-type mice when transgenic mice with labelled neurons are lacking.
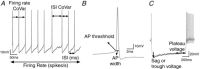
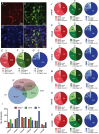
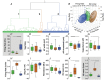
Abstract: Classification of the extensive neuronal diversity in the brain is fundamental for neuroscience. The globus pallidus external segment (GPe), also referred to as the globus pallidus in rodents, is a large nucleus located in the core of the basal ganglia whose circuitry is implicated in action control, decision-making and reward. Although considerable progress has been made in characterizing different GPe neuronal subtypes, no work has directly attempted to characterize these neurons in non-transgenic mice. Here, we provide data showing the degree of overlap in expression of neuronal PAS domain protein (Npas1), LIM homeobox 6 (Lhx6), parvalbumin (PV) and transcription factor FoxP2 biomarkers in mouse GPe neurons. We used an unbiased statistical method to classify neurons based on electrophysiological properties from nearly 200 neurons from C57BL/6J mice. In addition, we examined the subregion distribution of the neuronal subtypes. Cluster analysis using firing rate and hyperpolarization-induced membrane potential sag variables revealed three distinct neuronal clusters: type 1, characterized by low firing rate and small sag potential; type 2, with low firing rate and larger sag potential; and type 3, with high firing rate and small sag potential. We used other electrophysiological variables and data from marker-expressing neurons to evaluate the clusters. We propose that the GPe GABAergic neurons should be classified into three subgroups: arkypallidal, low-firing prototypical and high-firing prototypical neurons. This work will help researchers identify GPe neuron subtypes when transgenic mice with labelled neurons cannot be used.
Keywords: cluster analysis; electrophysiology signature; molecular signature.
Published 2018. This article is a U.S. Government work and is in the public domain in the USA.
Figures






References
-
- Abdi A, Mallet N, Mohamed FY, Sharott A, Dodson PD, Nakamura KC, Suri S, Avery SV, Larvin JT, Garas FN, Garas SN, Vinciati F, Morin S, Bezard E, Baufreton J & Magill PJ (2015). Prototypic and arkypallidal neurons in the dopamine‐intact external globus pallidus. J Neurosci 35, 6667–6688. - PMC - PubMed
-
- Albin RL, Young AB & Penney JB (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci 12, 366–375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

