Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters
- PMID: 29920186
- PMCID: PMC6118631
- DOI: 10.1172/JCI121309
Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters
Abstract
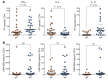
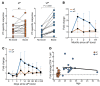
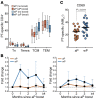
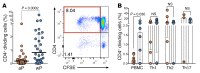
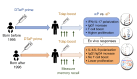
In the mid-1990s, whole-cell pertussis (wP) vaccines were associated with local and systemic adverse events that prompted their replacement with acellular pertussis (aP) vaccines in many high-income countries. In the past decade, rates of pertussis disease have increased in children receiving only aP vaccines. We compared the immune responses to aP boosters in individuals who received their initial doses with either wP or aP vaccines using activation-induced marker (AIM) assays. Specifically, we examined pertussis-specific memory CD4+ T cell responses ex vivo, highlighting a type 2/Th2 versus type 1/Th1 and Th17 differential polarization as a function of childhood vaccination. Remarkably, after a contemporary aP booster, cells from donors originally primed with aP were (a) associated with increased IL-4, IL-5, IL-13, IL-9, and TGF-β and decreased IFN-γ and IL-17 production, (b) defective in their ex vivo capacity to expand memory cells, and (c) less capable of proliferating in vitro. These differences appeared to be T cell specific, since equivalent increases of antibody titers and plasmablasts after aP boost were seen in both groups. In conclusion, our data suggest that there are long-lasting effects and differences in polarization and proliferation of T cell responses in adults originally vaccinated with aP compared with those that initially received wP, despite repeated acellular boosters.
Keywords: Adaptive immunity; Cellular immune response; Immunology; Th1 response; Vaccines.
Conflict of interest statement
Figures



Comment in
-
Composition of pertussis vaccine given to infants determines long-term T cell polarization.J Clin Invest. 2018 Aug 31;128(9):3742-3744. doi: 10.1172/JCI122726. Epub 2018 Aug 6. J Clin Invest. 2018. PMID: 30080178 Free PMC article.
References
-
- Epidemiology and Prevention of Vaccine-Preventable Diseases. CDC Website. https://www.cdc.gov/vaccines/pubs/pinkbook/pert.html Accessed June 22, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

