Analysis of short-term treatment with the phosphodiesterase type 5 inhibitor tadalafil on long bone development in young rats
- PMID: 29920215
- PMCID: PMC6230700
- DOI: 10.1152/ajpendo.00130.2018
Analysis of short-term treatment with the phosphodiesterase type 5 inhibitor tadalafil on long bone development in young rats
Abstract
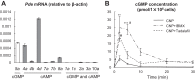
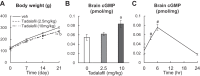
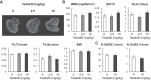
Cyclic GMP (cGMP) is an important intracellular regulator of endochondral bone growth and skeletal remodeling. Tadalafil, an inhibitor of the phosphodiesterase (PDE) type 5 (PDE5) that specifically hydrolyzes cGMP, is increasingly used to treat children with pulmonary arterial hypertension (PAH), but the effect of tadalafil on bone growth and strength has not been previously investigated. In this study, we first analyzed the expression of transcripts encoding PDEs in primary cultures of chondrocytes from newborn rat epiphyses. We detected robust expression of PDE5 as the major phosphodiesterase hydrolyzing cGMP. Time-course experiments showed that C-type natriuretic peptide increased intracellular levels of cGMP in primary chondrocytes with a peak at 2 min, and in the presence of tadalafil the peak level of intracellular cGMP was 37% greater ( P < 0.01) and the decline was significantly attenuated. Next, we treated 1-mo-old Sprague Dawley rats with vehicle or tadalafil for 3 wk. Although 10 mg·kg-1·day-1 tadalafil led to a significant 52% ( P < 0.01) increase in tissue levels of cGMP and a 9% reduction ( P < 0.01) in bodyweight gain, it did not alter long bone length, cortical or trabecular bone properties, and histological features. In conclusion, our results indicate that PDE5 is highly expressed in growth plate chondrocytes, and short-term tadalafil treatment of growing rats at doses comparable to those used in children with PAH has neither obvious beneficial effect on long bone growth nor any observable adverse effect on growth plate structure and trabecular and cortical bone structure.
Keywords: PDE5 inhibitor; bone; endochondral ossification; growth plate; young rat.
Figures


Similar articles
-
Cyclic GMP-dependent protein kinase II plays a critical role in C-type natriuretic peptide-mediated endochondral ossification.Endocrinology. 2002 Sep;143(9):3604-10. doi: 10.1210/en.2002-220307. Endocrinology. 2002. PMID: 12193576
-
Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development.Pediatr Res. 1998 Feb;43(2):163-8. doi: 10.1203/00006450-199802000-00002. Pediatr Res. 1998. PMID: 9475279
-
Phosphodiesterase 5 (PDE5) inhibition, ANP and NO rapidly reduce epididymal duct contractions, but long-term PDE5 inhibition in vivo does not.Mol Cell Endocrinol. 2012 Feb 26;349(2):145-53. doi: 10.1016/j.mce.2011.09.039. Epub 2011 Oct 2. Mol Cell Endocrinol. 2012. PMID: 21996373
-
Effects of tadalafil on skeletal muscle tissue: exploring interactions and novel mechanisms of action.Minerva Endocrinol (Torino). 2023 Jun;48(2):222-229. doi: 10.23736/S2724-6507.21.03698-8. Epub 2022 Feb 4. Minerva Endocrinol (Torino). 2023. PMID: 35119252 Review.
-
Tadalafil: a long-acting phosphodiesterase-5 inhibitor for the treatment of pulmonary arterial hypertension.Clin Ther. 2011 Aug;33(8):993-1004. doi: 10.1016/j.clinthera.2011.06.008. Epub 2011 Jul 16. Clin Ther. 2011. PMID: 21762988 Review.
Cited by
-
Repurposing erectile dysfunction drugs tadalafil and vardenafil to increase bone mass.Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14386-14394. doi: 10.1073/pnas.2000950117. Epub 2020 Jun 8. Proc Natl Acad Sci U S A. 2020. PMID: 32513693 Free PMC article.
-
Tadalafil and Steroid Hormones Interactions in Adipose, Bone and Prostate Tissues: Focus on Translational Perspectives.Int J Mol Sci. 2022 Apr 11;23(8):4191. doi: 10.3390/ijms23084191. Int J Mol Sci. 2022. PMID: 35457011 Free PMC article. Review.
References
-
- Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Erratum in Circulation 133: e368, 2016]. doi:10.1161/CIR.0000000000000329. - DOI - PubMed
-
- Ali Z, Schmidt P, Dodd J, Jeppesen DL. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan Med J 60: A4688, 2013. - PubMed
-
- Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, Ivy DD; STARTS-2 Investigators . STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 129: 1914–1923, 2014. doi:10.1161/CIRCULATIONAHA.113.005698. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

