Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer
- PMID: 29920275
- PMCID: PMC6011231
- DOI: 10.1016/j.devcel.2018.05.025
Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer
Abstract
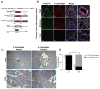
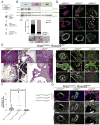
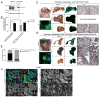
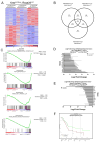
The regulation of metastatic organotropism in pancreatic ductal a denocarcinoma (PDAC) remains poorly understood. We demonstrate, using multiple mouse models, that liver and lung metastatic organotropism is dependent upon p120catenin (p120ctn)-mediated epithelial identity. Mono-allelic p120ctn loss accelerates KrasG12D-driven pancreatic cancer formation and liver metastasis. Importantly, one p120ctn allele is sufficient for E-CADHERIN-mediated cell adhesion. By contrast, cells with bi-allelic p120ctn loss demonstrate marked lung organotropism; however, rescue with p120ctn isoform 1A restores liver metastasis. In a p120ctn-independent PDAC model, mosaic loss of E-CADHERIN expression reveals selective pressure for E-CADHERIN-positive liver metastasis and E-CADHERIN-negative lung metastasis. Furthermore, human PDAC and liver metastases support the premise that liver metastases exhibit predominantly epithelial characteristics. RNA-seq demonstrates differential induction of pathways associated with metastasis and epithelial-to-mesenchymal transition in p120ctn-deficient versus p120ctn-wild-type cells. Taken together, P120CTN and E-CADHERIN mediated epithelial plasticity is an addition to the conceptual framework underlying metastatic organotropism in pancreatic cancer.
Keywords: E-cadherin; epithelial plasticity; metastasis; organotropism; p120catenin; p120catenin isoform; pancreatic cancer.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
EMT in Metastasis: Finding the Right Balance.Dev Cell. 2018 Jun 18;45(6):663-665. doi: 10.1016/j.devcel.2018.05.033. Dev Cell. 2018. PMID: 29920271
-
Epithelial plasticity is crucial for pancreatic cancer metastatic organotropism.Ann Transl Med. 2018 Nov;6(Suppl 1):S53. doi: 10.21037/atm.2018.10.16. Ann Transl Med. 2018. PMID: 30613628 Free PMC article. No abstract available.
References
-
- Aiello NM, Rhim AD, Stanger BZ. Orthotopic Injection of Pancreatic Cancer Cells. Cold Spring Harb Protoc. 2016b pdb.prot078360. - PubMed
-
- Aiello, et al. THIS ISSUE OF DEV CELL
-
- AmericanCancerSociety. American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

