Twist1 Activation in Muscle Progenitor Cells Causes Muscle Loss Akin to Cancer Cachexia
- PMID: 29920276
- PMCID: PMC6054474
- DOI: 10.1016/j.devcel.2018.05.026
Twist1 Activation in Muscle Progenitor Cells Causes Muscle Loss Akin to Cancer Cachexia
Abstract
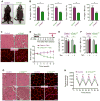
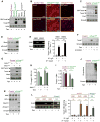
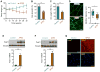
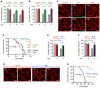
Cancer cachexia is characterized by extreme skeletal muscle loss that results in high morbidity and mortality. The incidence of cachexia varies among tumor types, being lowest in sarcomas, whereas 90% of pancreatic ductal adenocarcinoma (PDAC) patients experience severe weight loss. How these tumors trigger muscle depletion is still unfolding. Serendipitously, we found that overexpression of Twist1 in mouse muscle progenitor cells, either constitutively during development or inducibly in adult animals, caused severe muscle atrophy with features reminiscent of cachexia. Using several genetic mouse models of PDAC, we detected a marked increase in Twist1 expression in muscle undergoing cachexia. In cancer patients, elevated levels of Twist1 are associated with greater degrees of muscle wasting. Finally, both genetic and pharmacological inactivation of Twist1 in muscle progenitor cells afforded substantial protection against cancer-mediated cachexia, which translated into meaningful survival benefits, implicating Twist1 as a possible target for attenuating muscle cachexia in cancer patients.
Keywords: Activin/Myostatin cytokines; Smad signaling; Twist1; cancer cachexia; muscle cachexia; muscle progenitor cells; muscle protein breakdown; pancreatic ductal adenocarcinoma.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Chen ZF, Behringer RR. twist is required in head mesenchyme for cranial neural tube morphogenesis. Gene Dev. 1995;9:686–699. - PubMed
-
- Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. - PubMed
-
- Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

