Spatial regulation of signaling by the coordinated action of the protein tyrosine kinases MET and FER
- PMID: 29920310
- PMCID: PMC6530786
- DOI: 10.1016/j.cellsig.2018.06.006
Spatial regulation of signaling by the coordinated action of the protein tyrosine kinases MET and FER
Abstract
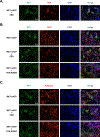
A critical aspect of understanding the regulation of signal transduction is not only to identify the protein-protein interactions that govern assembly of signaling pathways, but also to understand how those pathways are regulated in time and space. In this report, we have applied both gain-of-function and loss-of-function analyses to assess the role of the non-receptor protein tyrosine kinase FER in activation of the HGF Receptor protein tyrosine kinase MET. Overexpression of FER led to direct phosphorylation of several signaling sites in MET, including Tyr1349, but not the activation loop residues Tyr1234/5; in contrast, suppression of FER by RNAi revealed that phosphorylation of both a C-terminal signaling site (Tyr1349) and the activation loop (Tyr1234/5) were influenced by the function of this kinase. Adaptin β, a component of the adaptor protein complex 2 (AP-2) that links clathrin to receptors in coated vesicles, was recruited to MET following FER-mediated phosphorylation. Furthermore, we provide evidence to support a role of FER in maintaining plasma membrane distribution of MET and thereby delaying protein-tyrosine phosphatase PTP1B-mediated inactivation of the receptor. Simultaneous up-regulation of FER and down-regulation of PTP1B observed in ovarian carcinoma-derived cell lines would be expected to contribute to persistent activation of HGF-MET signaling, suggesting that targeting of both FER and MET may be an effective strategy for therapeutic intervention in ovarian cancer.
Keywords: Endocytosis; FER; MET; Ovarian cancer; PTP1B.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
All contributors declare that they have no financial or other conflicts of interest.
Figures

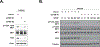




References
-
- Birchmeier C, Birchmeier W, Gherardi E and Vande Woude GF (2003) Met, metastasis, motility and more. Nature reviews. Molecular cell biology. 4, 915–925 - PubMed
-
- Trusolino L, Bertotti A and Comoglio PM (2010) MET signalling: principles and functions in development, organ regeneration and cancer. Nature reviews. Molecular cell biology. 11, 834–848 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

