IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice
- PMID: 29920352
- PMCID: PMC6298864
- DOI: 10.1016/j.jaci.2018.05.032
IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice
Erratum in
-
Corrigenda.J Allergy Clin Immunol. 2019 Oct;144(4):1142. doi: 10.1016/j.jaci.2019.07.026. J Allergy Clin Immunol. 2019. PMID: 31587795 No abstract available.
-
Corrigendum.J Allergy Clin Immunol. 2022 Jul;150(1):233. doi: 10.1016/j.jaci.2022.05.004. J Allergy Clin Immunol. 2022. PMID: 35803689 No abstract available.
Abstract
Background: Serum IL-22 levels are increased in patients with atopic dermatitis, which commonly precedes asthma in the atopic march. Epicutaneous sensitization in mice results in TH2-dominated skin inflammation that mimics atopic dermatitis and sensitizes the airways for antigen challenge-induced allergic inflammation characterized by the presence of both eosinophils and neutrophils. Epicutaneous sensitization results in increased serum levels of IL-22.
Objective: We sought to determine the role of IL-22 in antigen-driven airway allergic inflammation after inhalation challenge in epicutaneously sensitized mice.
Methods: Wild-type (WT) and Il22-/- mice were sensitized epicutaneously or immunized intraperitoneally with ovalbumin (OVA) and challenged intranasally with antigen. OVA T-cell receptor-specific T cells were TH22 polarized in vitro. Airway inflammation, mRNA levels in the lungs, and airway hyperresponsiveness (AHR) were examined.
Results: Epicutaneous sensitization preferentially elicited an IL-22 response compared with intraperitoneal immunization. Intranasal challenge of mice epicutaneously sensitized with OVA elicited in the lungs Il22 mRNA expression, IL-22 production, and accumulation of CD3+CD4+IL-22+ T cells that coexpressed IL-17A and TNF-α. Epicutaneously sensitized Il22-/- mice exhibited diminished eosinophil and neutrophil airway infiltration and decreased AHR after intranasal OVA challenge. Production of IL-13, IL-17A, and TNF-α was normal, but IFN-γ production was increased in lung cells from airway-challenged and epicutaneously sensitized Il22-/- mice. Intranasal instillation of IFN-γ-neutralizing antibody partially reversed the defect in eosinophil recruitment. WT recipients of TH22-polarized WT, but not IL-22-deficient, T-cell receptor OVA-specific T cells, which secrete both IL-17A and TNF-α, had neutrophil-dominated airway inflammation and AHR on intranasal OVA challenge. Intranasal instillation of IL-22 with TNF-α, but not IL-17A, elicited neutrophil-dominated airway inflammation and AHR in WT mice, suggesting that loss of IL-22 synergy with TNF-α contributed to defective recruitment of neutrophils into the airways of Il22-/- mice. TNF-α, but not IL-22, blockade at the time of antigen inhalation challenge inhibited airway inflammation in epicutaneously sensitized mice.
Conclusion: Epicutaneous sensitization promotes generation of antigen-specific IL-22-producing T cells that promote airway inflammation and AHR after antigen challenge, suggesting that IL-22 plays an important role in the atopic march.
Keywords: IL-22; asthma; atopic dermatitis; neutrophils.
Copyright © 2018 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
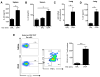
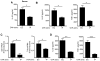

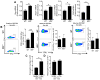



Similar articles
-
Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13.J Allergy Clin Immunol. 2009 Oct;124(4):761-70.e1. doi: 10.1016/j.jaci.2009.07.040. J Allergy Clin Immunol. 2009. PMID: 19815118 Free PMC article.
-
ST2 requires Th2-, but not Th17-, type airway inflammation in epicutaneously antigen- sensitized mice.Allergol Int. 2012 Jun;61(2):265-73. doi: 10.2332/allergolint.11-OA-0379. Epub 2012 Feb 25. Allergol Int. 2012. PMID: 22361513
-
CD1d restricted natural killer T cells are not required for allergic skin inflammation.J Allergy Clin Immunol. 2006 Dec;118(6):1363-8. doi: 10.1016/j.jaci.2006.08.010. Epub 2006 Sep 25. J Allergy Clin Immunol. 2006. PMID: 17157667
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Disease-associated functions of IL-33: the new kid in the IL-1 family.Nat Rev Immunol. 2010 Feb;10(2):103-10. doi: 10.1038/nri2692. Epub 2010 Jan 18. Nat Rev Immunol. 2010. PMID: 20081870 Review.
Cited by
-
Enhanced Type 2 Immune Reactions by Increased IL-22/IL-22Ra1 Signaling in Chronic Rhinosinusitis With Nasal Polyps.Allergy Asthma Immunol Res. 2020 Nov;12(6):980-993. doi: 10.4168/aair.2020.12.6.980. Allergy Asthma Immunol Res. 2020. PMID: 32935490 Free PMC article.
-
Role of IL-22 in persistent allergic airway diseases caused by house dust mite: a pilot study.BMC Pulm Med. 2021 Jan 21;21(1):36. doi: 10.1186/s12890-021-01410-z. BMC Pulm Med. 2021. PMID: 33478443 Free PMC article.
-
Interleukin-22 attenuates allergic airway inflammation in ovalbumin-induced asthma mouse model.BMC Pulm Med. 2021 Nov 26;21(1):385. doi: 10.1186/s12890-021-01698-x. BMC Pulm Med. 2021. PMID: 34836520 Free PMC article.
-
Interleukin-22 Contributes to Blood-Brain Barrier Disruption via STAT3/VEGFA Activation in Escherichia coli Meningitis.ACS Infect Dis. 2024 Mar 8;10(3):988-999. doi: 10.1021/acsinfecdis.3c00668. Epub 2024 Feb 6. ACS Infect Dis. 2024. PMID: 38317607 Free PMC article.
-
IgE Autoreactivity in Atopic Dermatitis: Paving the Road for Autoimmune Diseases?Antibodies (Basel). 2020 Sep 8;9(3):47. doi: 10.3390/antib9030047. Antibodies (Basel). 2020. PMID: 32911788 Free PMC article. Review.
References
-
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112:S118–27. - PubMed
-
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–94. - PubMed
-
- Di Cesare A, Di Meglio P, Nestle FO. A role for Th17 cells in the immunopathogenesis of atopic dermatitis? J Invest Dermatol. 2008;128:2569–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

