Improved de novo sequencing of heparin/heparan sulfate oligosaccharides by propionylation of sites of sulfation
- PMID: 29920400
- PMCID: PMC6286702
- DOI: 10.1016/j.carres.2018.06.002
Improved de novo sequencing of heparin/heparan sulfate oligosaccharides by propionylation of sites of sulfation
Abstract
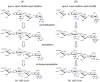
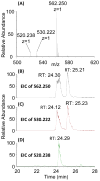
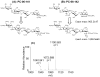
The structure of heparin and heparan sulfate (Hep/HS) oligosaccharides, as determined by the length and the pattern of sulfation, acetylation, and uronic acid epimerization, dictates their biological function through modulating interactions with protein targets. But fine structural determination is a very challenging task due to the lability of the sulfate modifications and difficulties in separating isomeric HS chains. Previously, we reported a strategy for chemical derivatization involving permethylation, desulfation, and trideuteroperacetylation, combined with standard reverse phase LC-MS/MS that enables the structural sequencing for heparin/HS oligosaccharides of sizes up to dodecasaccharide by positionally replacing all sulfates with more stable trideuteroacetyl groups, allowing for robust MS/MS sequencing. However, isomeric oligosaccharides that contain both N-sulfation and N-acetylation become isotopomers after labeling, differing only in the sites of deuteration. This prevents chromatographic separation of these different mixed domain sequences post-derivatization, and makes sequencing by MS/MS difficult due to co-fragmentation of the isotopomers leading to chimeric product ion spectra. In order to improve chromatographic separation of mixed domain oligosaccharides, we have introduced a propionylation step in place of trideuteroacetylation for labeling of sites of sulfation. HS standard disaccharides have been used to evaluate the efficiency of this improved chemical derivatization. The results show that we can quantitatively replace sulfation with propionyl groups with the same high efficiency as the previously reported trideuteroacetylation. After derivatization, we demonstrate the ability to chromatographically separate two mixed domain tetrasaccharide isomers differing solely by the order of N-sulfation and N-acetylation, allowing for full sequencing of each by MS/MS. These results represent a marked improvement in the ability of our previously reported derivatization strategy to analyze complex mixtures of Hep/HS oligosaccharides without a decrease in sensitivity.
Keywords: Chemical derivatization; Glycosaminoglycans; Heparin/heparan sulfate; LC-MS/MS.
Copyright © 2018. Published by Elsevier Ltd.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

