Increased susceptibility to oral Trichuris muris infection in the specific absence of CXCR5+ CD11c+ cells
- PMID: 29920694
- PMCID: PMC6099414
- DOI: 10.1111/pim.12566
Increased susceptibility to oral Trichuris muris infection in the specific absence of CXCR5+ CD11c+ cells
Abstract
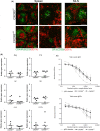
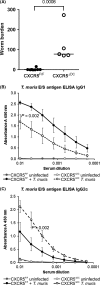
Trichuris muris is a natural mouse helminth pathogen which establishes infection specifically in the caecum and proximal colon. The rapid expulsion of T. muris in resistant mouse strains is associated with the induction of a protective T helper cell type 2 (Th2)-polarized immune response. Susceptible mouse strains, in contrast, mount an inappropriate Th1 response to T. muris infection. Expression of the chemokine CXCL13 by stromal follicular dendritic cells attracts CXCR5-expressing cells towards the B-cell follicles. Previous studies using a complex in vivo depletion model have suggested that CXCR5-expressing conventional dendritic cells (cDC) help regulate the induction of Th2-polarized responses. Here, transgenic mice with CXCR5 deficiency specifically restricted to CD11c+ cells were used to determine whether the specific absence CXCR5 on CD11c+ cells such as cDC would influence susceptibility to oral T. muris infection by affecting the Th1/Th2 balance. We show that in contrast to control mice, those which lacked CXCR5 expression on CD11c+ cells failed to clear T. muris infection and developed cytokine and antibody responses that suggested a disturbed Th1/Th2 balance with enhanced IFN-γ expression. These data suggest an important role of CXCR5-expressing CD11c+ cells such as cDC in immunity to oral T. muris infection.
Keywords: Trichuris muris; CXCR5; Th2; dendritic cell; helminth; mucosal immunity.
© 2018 The Authors. Parasite Immunology Published by John Wiley & Sons Ltd.
Figures



References
-
- Joeris T, Muller‐Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10:845‐864. - PubMed
-
- Persson EK, Uronen‐Hansson H, Semmrich M, et al. IRF4 transcription‐factor‐dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958‐969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F019726/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20231762/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/10002071/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/20002174/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

