Lactobacillus rhamnosus GG increases cyclooxygenase-2 expression and prostaglandin E2 secretion in colonic myofibroblasts via a MyD88-dependent mechanism during homeostasis
- PMID: 29920917
- PMCID: PMC6202218
- DOI: 10.1111/cmi.12871
Lactobacillus rhamnosus GG increases cyclooxygenase-2 expression and prostaglandin E2 secretion in colonic myofibroblasts via a MyD88-dependent mechanism during homeostasis
Erratum in
-
Erratum.Cell Microbiol. 2019 Aug;21(8):e13040. doi: 10.1111/cmi.13040. Epub 2019 May 21. Cell Microbiol. 2019. PMID: 31273929 No abstract available.
Abstract
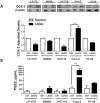
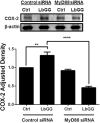
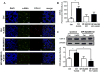
Prostaglandin E2 (PGE2 ) plays a critical role in intestinal mucosal tolerance and barrier integrity. Cyclooxygenase-2 (COX-2)-dependent PGE2 production involves mobilisation of arachidonic acid. Lactobacillus rhamnosus GG (LbGG) is one of the most widely used probiotics reported to colonise the colonic mucosa. LbGG contributes to the protection of the small intestine against radiation injury through the repositioning of mucosal COX-2 expressing cells. However, it is unknown if LbGG modulates PGE2 production in the colonic mucosa under homeostasis and the major cellular elements involved in these processes. Colonic epithelial and CD90+ mesenchymal stromal cells, also known as (myo) fibroblasts (CMFs), are abundant innate immune cells in normal colonic mucosa able to produce PGE2 . Herein, we tested the hypothesis that under colonic mucosal homeostasis, LbGG modulates the eicosanoid pathway resulting in increased PGE2 production in both epithelial and stromal cells. Among the five tested human colonic epithelial cell lines, only exposure of Caco-2 to LbGG for 24 hr led to the mobilisation of arachidonic acid with concomitant increase in the components within the leukotriene and COX-2-dependent PGE2 pathways. By contrast, CMFs isolated from the normal human colonic mucosa responded to LbGG with increased expression of COX-2 and PGE2 in the prostaglandin pathway, but not 5-LO in the leukotriene pathway. Oral gavage of C57BL/6 mice for 5 days with LbGG (5 × 108 Colony-Forming Unit (CFU)/dose) increased COX-2 expression in the colonic mucosa. The majority of cells upregulating COX-2 protein expression were located in the colonic lamina propria and colocalised with α-SMA+ cells corresponding to the CMF phenotype. This process was myeloid differentiation factor-88-dependent, because silencing of myeloid differentiation factor-88 expression in CMFs abrogated LbGG-induced upregulation of COX-2 in culture and in vivo. Taken together, our data suggest that LbGG increases release of COX-2-mediated PGE2 , contributing to the maintenance of mucosal homeostasis in the colon and CMFs are among the major contributors to this process.
Keywords: COX-2; lactic acid bacteria; mechanism of action; metabolic processes; microbial cell interaction.
© 2018 John Wiley & Sons Ltd.
Conflict of interest statement
Figures



Similar articles
-
TLR4 activation enhances the PD-L1-mediated tolerogenic capacity of colonic CD90+ stromal cells.J Immunol. 2014 Sep 1;193(5):2218-29. doi: 10.4049/jimmunol.1203441. Epub 2014 Jul 28. J Immunol. 2014. PMID: 25070848 Free PMC article. Clinical Trial.
-
Defective arachidonate release and PGE2 production in Gi alpha2-deficient intestinal and colonic subepithelial myofibroblasts.Inflamm Bowel Dis. 2006 Mar;12(3):153-65. doi: 10.1097/01.MIB.0000201100.72191.19. Inflamm Bowel Dis. 2006. PMID: 16534415
-
Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner.Gut. 2012 Jun;61(6):829-38. doi: 10.1136/gutjnl-2011-300367. Epub 2011 Oct 24. Gut. 2012. PMID: 22027478 Free PMC article.
-
Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.Cancer Sci. 2016 Apr;107(4):391-7. doi: 10.1111/cas.12901. Epub 2016 Mar 18. Cancer Sci. 2016. PMID: 27079437 Free PMC article. Review.
-
Role of the Cyclooxygenase Pathway in the Association of Obstructive Sleep Apnea and Cancer.J Clin Med. 2020 Oct 10;9(10):3237. doi: 10.3390/jcm9103237. J Clin Med. 2020. PMID: 33050416 Free PMC article. Review.
Cited by
-
Angiogenin regulates PKD activation and COX-2 expression induced by TNF-α and bradykinin in the colonic myofibroblast.Biochem Biophys Res Commun. 2020 May 14;525(4):870-876. doi: 10.1016/j.bbrc.2020.02.169. Epub 2020 Mar 12. Biochem Biophys Res Commun. 2020. PMID: 32171525 Free PMC article.
-
Role of prostaglandin E2 in tissue repair and regeneration.Theranostics. 2021 Aug 13;11(18):8836-8854. doi: 10.7150/thno.63396. eCollection 2021. Theranostics. 2021. PMID: 34522214 Free PMC article. Review.
-
Nonmicrobial Activation of TLRs Controls Intestinal Growth, Wound Repair, and Radioprotection.Front Immunol. 2021 Jan 21;11:617510. doi: 10.3389/fimmu.2020.617510. eCollection 2020. Front Immunol. 2021. PMID: 33552081 Free PMC article. Review.
-
Gastric mucosal repair by Men's Huwei Powder via EGF-NO/PGE2-PI3K-TLR4 in RELISH: Restoring Equilibrium through long-term integration of synergistic health.Front Pharmacol. 2025 Jul 11;16:1594089. doi: 10.3389/fphar.2025.1594089. eCollection 2025. Front Pharmacol. 2025. PMID: 40717975 Free PMC article.
-
Biotechnological Processes Simulating the Natural Fermentation Process of Bee Bread and Therapeutic Properties-An Overview.Front Nutr. 2022 Apr 27;9:871896. doi: 10.3389/fnut.2022.871896. eCollection 2022. Front Nutr. 2022. PMID: 35571893 Free PMC article. Review.
References
-
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. The Journal of clinical investigation. 2007;117(1):258–269. doi: 10.1172/jci29159. - DOI - PMC - PubMed
-
- Buecher B, Bouancheau D, Broquet A, Bezieau S, Denis MG, Bonnet C, Heymann MF, Jarry A, Galmiche JP, Blottière HM. Growth Inhibitory Effect of Celecoxib and Rofecoxib on Human Colorectal Carcinoma Cell Lines. Anticancer Research. 2005;25(1A):225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

