PBPK Model of Morphine Incorporating Developmental Changes in Hepatic OCT1 and UGT2B7 Proteins to Explain the Variability in Clearances in Neonates and Small Infants
- PMID: 29920988
- PMCID: PMC6063742
- DOI: 10.1002/psp4.12306
PBPK Model of Morphine Incorporating Developmental Changes in Hepatic OCT1 and UGT2B7 Proteins to Explain the Variability in Clearances in Neonates and Small Infants
Abstract
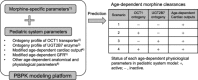
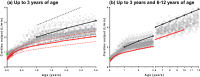
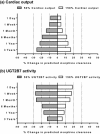
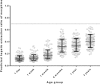
Morphine has large pharmacokinetic variability, which is further complicated by developmental changes in neonates and small infants. The impacts of organic cation transporter 1 (OCT1) genotype and changes in blood-flow on morphine clearance (CL) were previously demonstrated in children, whereas changes in UDP-glucuronosyltransferase 2B7 (UGT2B7) activity showed a small effect. This study, targeting neonates and small infants, was designed to assess the influence of developmental changes in OCT1 and UGT2B7 protein expression and modified blood-flow on morphine CL using physiologically based pharmacokinetic (PBPK) modeling. The implementation of these three age-dependent factors into the pediatric system platform resulted in reasonable prediction for an age-dependent increase in morphine CL in these populations. Sensitivity of morphine CL to changes in cardiac output increased with age up to 3 years, whereas sensitivity to changes in UGT2B7 activity decreased. This study suggests that morphine exhibits age-dependent extraction, likely due to the developmental increase in OCT1 and UGT2B7 protein expression/activity and hepatic blood-flow.
© 2018 The Authors CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures


References
-
- Bion, J.F. et al Sedation in intensive care: morphine and renal function. Intensive Care Med. 12, 359–365 (1986). - PubMed
-
- Nelson, K.L. , Yaster, M. , Kost‐Byerly, S. & Monitto, C.L. A national survey of American Pediatric Anesthesiologists: patient‐controlled analgesia and other intravenous opioid therapies in pediatric acute pain management. Anesth. Analg 110, 754–760 (2010). - PubMed
-
- Tolia, V.N. et al Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N. Engl. J. Med. 372, 2118–2126 (2015). - PubMed
-
- Anderson, B.J. & Holford, N.H. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab. Pharmacokinet. 24, 25–36 (2009). - PubMed
-
- Vinks, A.A. , Emoto, C. & Fukuda, T. Modeling and simulation in pediatric drug therapy: application of pharmacometrics to define the right dose for children. Clin. Pharmacol. Ther. 98, 298–308 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

