Is intra-bladder pressure measurement a reliable indicator for raised intra-abdominal pressure? A prospective comparative study
- PMID: 29921222
- PMCID: PMC6009941
- DOI: 10.1186/s12871-018-0539-z
Is intra-bladder pressure measurement a reliable indicator for raised intra-abdominal pressure? A prospective comparative study
Abstract
Background: Intra-abdominal pressure (IAP) can be measured by several indirect methods; however, the urinary bladder is largely preferred. The aim of this study was to compare intra-bladder pressure (IBP) at different levels of IAPs and assess its reliability as an indirect method for IAP measurement.
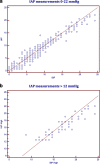
Methods: We compared IBP with IAP in twenty-one patients undergoing laparoscopic cholecystectomy under general anesthesia. Measurements were recorded at increasing levels of insufflation pressures to approximately 22 mmHg. Pearson's correlation coefficient was calculated to establish the relationship between the two pressure measurements and Bland-Altman analysis was used to assess the limits of agreement between the two methods of measurements.
Results: The urinary bladder pressures reflected well the pressures in the abdominal cavity. Pearson correlation coefficient showed a good correlation between the two measurement techniques (r = 0.966, p < 0.0001) and Bland-Altman analysis indicated that the 95% limits of agreement between the two methods ranged from - 2.83 to 2.64. This range is accepted both clinically and according to the recommendations of the World Society of Abdominal Compartment Syndrome (WSACS).
Conclusion: Our study showed that IBP measurement is a simple, minimally invasive method that may reliably estimates IAP in patients placed in supine position. Measurements for pressures higher than 12 mmHg may be less reliable. When applied clinically, this should alert the clinician to take safety measures to avoid abdominal compartment syndrome (ACS).
Keywords: Compartment syndrome; Intra-abdominal hypertension; Intra-abdominal pressure; Intra-vesical pressure; Urinary bladder pressure.
Conflict of interest statement
Ethics approval and consent to participate
This study was conducted at Saqr Hospital, Ras Al Khaimah, United Arab Emirates, in accordance with the study protocol approved by the hospital research ethics committee. All patients consented for participation in the study and agreed for publishing study results in medical journals.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials