Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison
- PMID: 29921311
- PMCID: PMC6008936
- DOI: 10.1186/s13287-018-0914-1
Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison
Abstract
Background: Adipose-derived stem cells (ASCs) have been introduced as an alternative to bone marrow mesenchymal stem cells (BMSCs) for cell-based therapy. However, different studies comparing ASCs and BMSCs have shown conflicting results. In fact, harvesting ASCs and BMSCs from different individuals might influence the results, making comparison difficult. Therefore, this study aimed to characterize donor-matched ASCs and BMSCs in order to investigate proliferation, differentiation potential and possible effects of donor variation on these mesenchymal stem cells (MSCs).
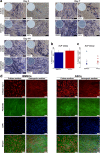
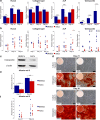
Methods: Human bone marrow and adipose tissue samples were obtained from nine donors aged 8-14. ASCs and BMSCs were isolated and characterized based on expression of surface markers using flow cytometry. The proliferation up to 21 days was investigated. Multi-lineage differentiation was induced using osteogenic, chondrogenic and adipogenic differentiation media. Alkaline phosphatase (ALP) activity was monitored and collagen type I formation was evaluated by immunofluorescence staining. In vitro multi-potency was studied using tissue-specific stains and lineage-specific gene expression. In addition, the osteogenic lineage was evaluated at protein level.
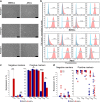
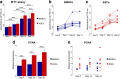
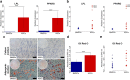
Results: Isolated ASCs and BMSCs from all donors demonstrated morphologic and immunophenotypic characteristics of MSCs, with expression of MSCs markers and negative expression of hematopoietic markers. Unlike BMSCs, ASCs showed high expression of CD49d and low expression of Stro-1. In general, ASCs showed significantly higher proliferation and adipogenic capacity with more lipid vesicle formation and expression of the adipogenesis-related genes than BMSCs. In contrast, BMSCs showed significantly higher osteogenic and chondrogenic capacity compared to ASCs. BMSCs had earlier and higher ALP activity, calcium deposition, and expression of the osteogenesis- and chondrogenesis-related genes and the osteogenesis-related protein osteopontin. Proliferation and differentiation capacity of ASCs and BMSCs varied significantly among the donors.
Conclusions: ASCs and BMSCs showed tissue-specific differentiation abilities, but with significant variation between donors. The similarities and differences in the properties of ASCs and BMSCs should be taken into consideration when planning stem cell-based therapy.
Keywords: Adipose-derived stem cells; Bone marrow mesenchymal stem cells; Characterization; Differentiation; Proliferation.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for this study was obtained from the Regional Committees for Medical and Health Research Ethics (REK) in Norway (Reference number: 2013/1248/REK sør-øst C). All samples were obtained with informed parental consent.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
Tissue source determines the differentiation potentials of mesenchymal stem cells: a comparative study of human mesenchymal stem cells from bone marrow and adipose tissue.Stem Cell Res Ther. 2017 Dec 6;8(1):275. doi: 10.1186/s13287-017-0716-x. Stem Cell Res Ther. 2017. PMID: 29208029 Free PMC article.
-
Donor-matched mesenchymal stem cells from knee infrapatellar and subcutaneous adipose tissue of osteoarthritic donors display differential chondrogenic and osteogenic commitment.Eur Cell Mater. 2014 Apr 23;27:298-311. doi: 10.22203/ecm.v027a21. Eur Cell Mater. 2014. PMID: 24760577
-
Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro.Tissue Eng. 2007 Jan;13(1):111-21. doi: 10.1089/ten.2006.0114. Tissue Eng. 2007. PMID: 17518585
-
Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells.World J Stem Cells. 2014 Jul 26;6(3):288-95. doi: 10.4252/wjsc.v6.i3.288. World J Stem Cells. 2014. PMID: 25126378 Free PMC article. Review.
-
Osteogenic Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells on Composite Polymeric Scaffolds: A Review.Curr Stem Cell Res Ther. 2025;20(1):33-49. doi: 10.2174/011574888X263333231218065453. Curr Stem Cell Res Ther. 2025. PMID: 38315659 Review.
Cited by
-
Bone defect reconstruction via endochondral ossification: A developmental engineering strategy.J Tissue Eng. 2021 Mar 30;12:20417314211004211. doi: 10.1177/20417314211004211. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 33868628 Free PMC article. Review.
-
BRD4 facilitates osteogenic differentiation of human bone marrow mesenchymal stem cells through WNT4/NF-κB pathway.J Orthop Surg Res. 2023 Nov 18;18(1):876. doi: 10.1186/s13018-023-04335-x. J Orthop Surg Res. 2023. PMID: 37980502 Free PMC article.
-
Mesenchymal stem cell therapy for neurological disorders: The light or the dark side of the force?Front Bioeng Biotechnol. 2023 Feb 28;11:1139359. doi: 10.3389/fbioe.2023.1139359. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36926687 Free PMC article. Review.
-
Heat Shock Protein 27 Is Involved in the Bioactive Glass Induced Osteogenic Response of Human Mesenchymal Stem Cells.Cells. 2023 Jan 5;12(2):224. doi: 10.3390/cells12020224. Cells. 2023. PMID: 36672159 Free PMC article.
-
Comparative Study of the Osteogenic Differentiation Potential of Adipose Tissue-Derived Stromal Cells and Dedifferentiated Adipose Cells of the Same Tissue Origin under Pro and Antioxidant Conditions.Biomedicines. 2022 Nov 29;10(12):3071. doi: 10.3390/biomedicines10123071. Biomedicines. 2022. PMID: 36551827 Free PMC article.
References
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

