Inhibition of the RhoGTPase Cdc42 by ML141 enhances hepatocyte differentiation from human adipose-derived mesenchymal stem cells via the Wnt5a/PI3K/miR-122 pathway: impact of the age of the donor
- PMID: 29921325
- PMCID: PMC6009972
- DOI: 10.1186/s13287-018-0910-5
Inhibition of the RhoGTPase Cdc42 by ML141 enhances hepatocyte differentiation from human adipose-derived mesenchymal stem cells via the Wnt5a/PI3K/miR-122 pathway: impact of the age of the donor
Abstract
Background: Human adipose-derived mesenchymal stem cells (hADSCs) are promising cells that may promote hepatocyte differentiation (Hep-Dif) and improve liver function, but the involvement of Cdc42, a key small RhoGTPase which plays a crucial role in aging, is still not well established. We hypothesized that the inhibition of Cdc42 may rescue the hepatogenic potential of hADSCs derived from aged donors.
Methods: hADSCs isolated from 61 women of different ages were cultured for evaluation of the proliferation of cells, adherence, apoptosis, immunomodulation, immunophenotyping, multipotency, gene expression, and cell function during Hep-Dif. Inhibition of Cdc42 by ML141 was realized during two phases: initiation (days -2 to 14 (D-2/14)) from undifferentiated to hepatoblast-like cells, or maturation (days 14 to 28 (D14/28)) from undifferentiated to hepatocyte-like cells. Mechanistic insights of the Wnt(s)/MAPK/PI3K/miR-122 pathways were studied.
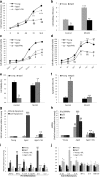
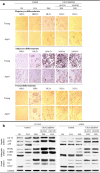
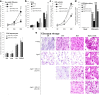
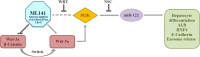
Results: Cdc42 activity in undifferentiated hADSCs showed an age-dependent significant increase in Cdc42-GTP correlated to a decrease in Cdc42GAP; the low potentials of cell proliferation, doubling, adherence, and immunomodulatory ability (proinflammatory over anti-inflammatory) contrary to the apoptotic index of the aged group were significantly reversed by ML141. Aged donor cells showed a decreased potential for Hep-Dif which was rescued by ML141 treatment, giving rise to mature and functional hepatocyte-like cells as assessed by hepatic gene expression, cytochrome activity, urea and albumin production, low-density lipoprotein (LDL) uptake, and glycogen storage. ML141-induced Hep-Dif showed an improvement in mesenchymal-epithelial transition, a switch from Wtn-3a/β-catenin to Wnt5a signaling, involvement of PI3K/PKB but not the MAPK (ERK/JNK/p38) pathway, induction of miR-122 expression, reinforcing the exosomes release and the production of albumin, and epigenetic changes. Inhibition of PI3K and miR-122 abolished completely the effects of ML141 indicating that inhibition of Cdc42 promotes the Hep-Dif through a Wnt5a/PI3K/miR-122/HNF4α/albumin/E-cadherin-positive action. The ML141(D-2/14) protocol had more pronounced effects when compared with ML141(D14/28); inhibition of DNA methylation in combination with ML141(D-2/14) showed more efficacy in rescuing the Hep-Dif of aged hADSCs. In addition to Hep-Dif, the multipotency of aged hADSC-treated ML141 was observed by rescuing the adipocyte and neural differentiation by inducing PPARγ/FABP4 and NeuN/O4 but inhibiting Pref-1 and GFAP, respectively.
Conclusion: ML141 has the potential to reverse the age-related aberrations in aged stem cells and promotes their hepatogenic differentiation. Selective inhibition of Cdc42 could be a potential target of drug therapy for aging and may give new insights on the improvement of Hep-Dif.
Keywords: Adipose derived mesenchymal stem cells; Aging; Cdc42; Exosomes release; Hepatocyte differentiation; MAPK; ML141; PI3K; Wnt; miR122.
Conflict of interest statement
Ethics approval and consent to participate
All clinical investigations on human samples have been conducted according to the principles expressed in the Declaration of Helsinki (
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

