IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1α and VEGF Expression in Vascular Endothelial Cells
- PMID: 29921553
- PMCID: PMC6085568
- DOI: 10.1016/j.ebiom.2018.06.003
IL-33 Initiates Vascular Remodelling in Hypoxic Pulmonary Hypertension by up-Regulating HIF-1α and VEGF Expression in Vascular Endothelial Cells
Abstract
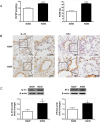
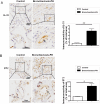
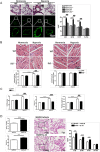
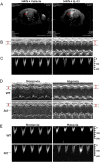
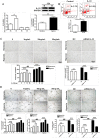
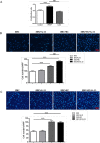
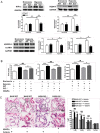
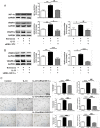
IL-33 may play a role in the vascular remodelling of hypoxic pulmonary hypertension (PH) but the precise mechanisms are still unclear. We hypothesized that hypoxia promotes expression of IL-33 and its receptor ST2 on vascular endothelial cells, which in turn leads to dysfunction of vascular endothelial cells and smooth muscle cells contributing to PH. Immunohistochemistry showed that immunoreactivity for IL-33 and ST2 was significantly increased in lung tissue of murine model of hypoxia-induced PH (HPH) and of subjects with bronchiectasis-PH. trans-Thoracic echocardiography showed that haemodynamic changes and right ventricular hypertrophy associated with HPH were significantly abrogated in St2-/- compared with WT mice. Administration of IL-33 further exacerbated these changes in the hypoxia-exposed WT mice. In vitro, hypoxia significantly increased IL-33/ST2 expression by human pulmonary arterial endothelial cells (HPAECs), while exogenous IL-33 enhanced proliferation, adhesiveness and spontaneous angiogenesis of HPAECs. Knockdown of endogenous Il33 or St2 using siRNA transfection significantly suppressed these effects in both normoxic and hypoxic culture-conditions. Deletion of the St2 gene attenuated hypoxia-induced, elevated lung expression of HIF-1α/VEGFA/VEGFR-2/ICAM-1, while administration of exogenous VEGFA partially reversed the attenuation of the haemodynamic indices of PH. Correspondingly, knockdown of the St2 or Hif1α genes almost completely abrogated IL-33-induced expression of HIF-1α/VEGFA/VEGFR-2 by HPAECs in vitro. Further, IL-33-induced angiogenesis by HPAECs was extensively abrogated by knockdown of the Hif1α/Vegfa or Vegfr2 genes. These data suggest that hypoxia induces elevated expression of IL-33/ST2 by HPAECs which, at least partly by increasing downstream expression of HIF-1α and VEGF initiates vascular remodelling resulting in HPH.
Keywords: Hypoxia inducible factor 1; Hypoxic pulmonary hypertension; IL-33; Vascular endothelial growth factor.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
IL-33-HIF1α Axis in Hypoxic Pulmonary Hypertension.EBioMedicine. 2018 Jul;33:8-9. doi: 10.1016/j.ebiom.2018.07.004. Epub 2018 Jul 7. EBioMedicine. 2018. PMID: 30049389 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

