Acute Complement Inhibition Potentiates Neurorehabilitation and Enhances tPA-Mediated Neuroprotection
- PMID: 29921716
- PMCID: PMC6052238
- DOI: 10.1523/JNEUROSCI.0111-18.2018
Acute Complement Inhibition Potentiates Neurorehabilitation and Enhances tPA-Mediated Neuroprotection
Abstract
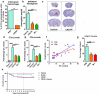
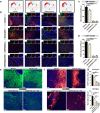
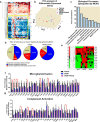
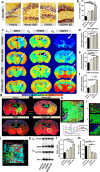
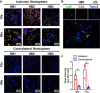
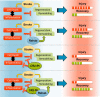
Because complement activation in the subacute or chronic phase after stroke was recently shown to stimulate neural plasticity, we investigated how complement activation and complement inhibition in the acute phase after murine stroke interacts with subsequent rehabilitation therapy to modulate neuroinflammation and neural remodeling. We additionally investigated how complement and complement inhibition interacts with tissue plasminogen activator (tPA), the other standard of care therapy for stroke, and a U.S. Food and Drug Administration preclinical requirement for translation of an experimental stroke therapy. CR2fH, an injury site-targeted inhibitor of the alternative complement pathway, significantly reduced infarct volume, hemorrhagic transformation, and mortality and significantly improved long-term motor and cognitive performance when administered 1.5 or 24 h after middle cerebral artery occlusion. CR2fH interrupted a poststroke inflammatory process and significantly reduced inflammatory cytokine release, microglial activation, and astrocytosis. Rehabilitation alone showed mild anti-inflammatory effects, including reduced complement activation, but only improved cognitive recovery. CR2fH combined with rehabilitation significantly potentiated cognitive and motor recovery compared with either intervention alone and was associated with higher growth factor release and enhanced rehabilitation-induced neuroblast migration and axonal remodeling. Similar outcomes were seen in adult, aged, and female mice. Using a microembolic model, CR2fH administered in combination with acute tPA therapy improved overall survival and enhanced the neuroprotective effects of tPA, extending the treatment window for tPA therapy. A human counterpart of CR2fH has been shown to be safe and nonimmunogenic in humans and we have demonstrated robust deposition of C3d, the CR2fH targeting epitope, in ischemic human brains after stroke.SIGNIFICANCE STATEMENT Complement inhibition is a potential therapeutic approach for stroke, but it is not known how complement inhibition would interact with current standards of care. We show that, after murine ischemic stroke, rehabilitation alone induced mild anti-inflammatory effects and improved cognitive, but not motor recovery. However, brain-targeted and specific inhibition of the alternative complement pathway, when combined with rehabilitation, significantly potentiated cognitive and motor recovery compared with either intervention alone via mechanisms involving neuroregeneration and enhanced brain remodeling. Further, inhibiting the alternative pathway of complement significantly enhanced the neuroprotective effects of thrombolytic therapy and markedly expanded the therapeutic window for thrombolytic therapy.
Keywords: complement; rehabilitation; thrombolysis.
Copyright © 2018 the authors 0270-6474/18/386527-19$15.00/0.
Figures





References
-
- Alawieh A, Elvington A, Zhu H, Yu J, Kindy MS, Atkinson C, Tomlinson S (2015b) Modulation of post-stroke degenerative and regenerative processes and subacute protection by site-targeted inhibition of the alternative pathway of complement. J Neuroinflammation 12:247. 10.1186/s12974-015-0464-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
