Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering
- PMID: 29921804
- PMCID: PMC6032265
- DOI: 10.3390/ijms19061807
Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering
Abstract
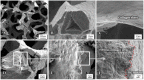
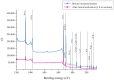
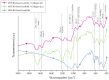
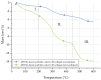
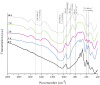
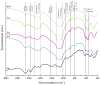
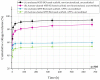
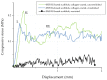
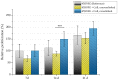
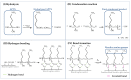
Highly porous 45S5 bioactive glass-based scaffolds were fabricated by the foam replica technique and coated with collagen by a novel method. After an initial cleaning step of the bioactive glass surface to expose reactive –OH groups, samples were surface functionalized by (3-aminopropyl)triethoxysilane (APTS). Functionalized scaffolds were immersed in a collagen solution, left for gelling at 37 °C, and dried at room temperature. The collagen coating was further stabilized by crosslinking with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS). Applying this coating method, a layer thickness of a few micrometers was obtained without affecting the overall scaffold macroporosity. In addition, values of compressive strength were enhanced by a factor of five, increasing from 0.04 ± 0.02 MPa for uncoated scaffolds to 0.18 ± 0.03 MPa for crosslinked collagen-coated scaffolds. The composite material developed in this study exhibited positive cell (MG-63) viability as well as suitable cell attachment and proliferation on the surface. The combination of bioactivity, mechanical competence, and cellular response makes this novel scaffold system attractive for bone tissue engineering.
Keywords: bioactive glass; bone tissue engineering; collagen; scaffolds; surface functionalization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications.Colloids Surf B Biointerfaces. 2019 Oct 1;182:110346. doi: 10.1016/j.colsurfb.2019.110346. Epub 2019 Jul 4. Colloids Surf B Biointerfaces. 2019. PMID: 31325780
-
Biomimetic component coating on 3D scaffolds using high bioactivity of mesoporous bioactive ceramics.Int J Nanomedicine. 2011;6:2521-31. doi: 10.2147/IJN.S25647. Epub 2011 Oct 21. Int J Nanomedicine. 2011. PMID: 22072886 Free PMC article.
-
In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds.Acta Biomater. 2016 Jan;30:319-333. doi: 10.1016/j.actbio.2015.11.012. Epub 2015 Nov 10. Acta Biomater. 2016. PMID: 26563472
-
Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration: a review of the last 5 years.Expert Rev Med Devices. 2015 Jan;12(1):93-111. doi: 10.1586/17434440.2015.958075. Epub 2014 Oct 21. Expert Rev Med Devices. 2015. PMID: 25331196 Review.
-
Bioglass® 45S5-based composites for bone tissue engineering and functional applications.J Biomed Mater Res A. 2017 Nov;105(11):3197-3223. doi: 10.1002/jbm.a.36156. Epub 2017 Aug 9. J Biomed Mater Res A. 2017. PMID: 28686004 Review.
Cited by
-
Current and Future Perspectives of Bioactive Glasses as Injectable Material.Nanomaterials (Basel). 2024 Jul 13;14(14):1196. doi: 10.3390/nano14141196. Nanomaterials (Basel). 2024. PMID: 39057873 Free PMC article. Review.
-
Wound healing strategies based on nanoparticles incorporated in hydrogel wound patches.RSC Adv. 2023 Jul 17;13(31):21345-21364. doi: 10.1039/d3ra03477a. eCollection 2023 Jul 12. RSC Adv. 2023. PMID: 37465579 Free PMC article. Review.
-
Factors Influencing Properties of Spider Silk Coatings and Their Interactions within a Biological Environment.J Funct Biomater. 2023 Aug 19;14(8):434. doi: 10.3390/jfb14080434. J Funct Biomater. 2023. PMID: 37623678 Free PMC article. Review.
-
Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering.Materials (Basel). 2018 Dec 12;11(12):2530. doi: 10.3390/ma11122530. Materials (Basel). 2018. PMID: 30545136 Free PMC article. Review.
-
Mesoporous Silica-Bioglass Composite Pellets as Bone Drug Delivery System with Mineralization Potential.Int J Mol Sci. 2021 Apr 29;22(9):4708. doi: 10.3390/ijms22094708. Int J Mol Sci. 2021. PMID: 33946793 Free PMC article.
References
-
- Yunos D.M., Bretcanu O., Boccaccini A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008;43:4433–4442. doi: 10.1007/s10853-008-2552-y. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

