Caffeine-inducible gene switches controlling experimental diabetes
- PMID: 29921872
- PMCID: PMC6008335
- DOI: 10.1038/s41467-018-04744-1
Caffeine-inducible gene switches controlling experimental diabetes
Abstract
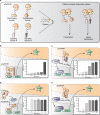
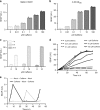
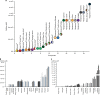
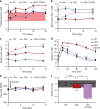
Programming cellular behavior using trigger-inducible gene switches is integral to synthetic biology. Although significant progress has been achieved in trigger-induced transgene expression, side-effect-free remote control of transgenes continues to challenge cell-based therapies. Here, utilizing a caffeine-binding single-domain antibody we establish a caffeine-inducible protein dimerization system, enabling synthetic transcription factors and cell-surface receptors that enable transgene expression in response to physiologically relevant concentrations of caffeine generated by routine intake of beverages such as tea and coffee. Coffee containing different caffeine concentrations dose-dependently and reversibly controlled transgene expression by designer cells with this caffeine-stimulated advanced regulators (C-STAR) system. Type-2 diabetic mice implanted with microencapsulated, C-STAR-equipped cells for caffeine-sensitive expression of glucagon-like peptide 1 showed substantially improved glucose homeostasis after coffee consumption compared to untreated mice. Biopharmaceutical production control by caffeine, which is non-toxic, inexpensive and only present in specific beverages, is expected to improve patient compliance by integrating therapy with lifestyle.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Construction of Caffeine-Inducible Gene Switches in Mammalian Cells.Methods Mol Biol. 2021;2312:159-168. doi: 10.1007/978-1-0716-1441-9_10. Methods Mol Biol. 2021. PMID: 34228290
-
A synthetic free fatty acid-regulated transgene switch in mammalian cells and mice.Nucleic Acids Res. 2018 Oct 12;46(18):9864-9874. doi: 10.1093/nar/gky805. Nucleic Acids Res. 2018. PMID: 30219861 Free PMC article.
-
Coffee and caffeine improve insulin sensitivity and glucose tolerance in C57BL/6J mice fed a high-fat diet.Biosci Biotechnol Biochem. 2011;75(12):2309-15. doi: 10.1271/bbb.110452. Epub 2011 Dec 7. Biosci Biotechnol Biochem. 2011. PMID: 22146708
-
[Coffee consumption and the decreased risk of diabetes mellitus type 2].Ned Tijdschr Geneeskd. 2006 Aug 19;150(33):1821-5. Ned Tijdschr Geneeskd. 2006. PMID: 16967592 Review. Dutch.
-
Teratogen update: evaluation of the reproductive and developmental risks of caffeine.Teratology. 2001 Jul;64(1):51-78. doi: 10.1002/tera.1047. Teratology. 2001. PMID: 11410911 Review.
Cited by
-
Design and characterization of a salicylic acid-inducible gene expression system for Jurkat cells.J Biotechnol. 2022 Feb 20;346:11-14. doi: 10.1016/j.jbiotec.2022.01.003. Epub 2022 Jan 18. J Biotechnol. 2022. PMID: 35051448 Free PMC article.
-
The transcription factor CsS40 negatively regulates TCS1 expression and caffeine biosynthesis in connection to leaf senescence in Camellia sinensis.Hortic Res. 2023 Aug 10;10(9):uhad162. doi: 10.1093/hr/uhad162. eCollection 2023 Sep. Hortic Res. 2023. PMID: 37731861 Free PMC article.
-
Synthetic Receptors for Sensing Soluble Molecules with Mammalian Cells.Methods Mol Biol. 2021;2312:15-33. doi: 10.1007/978-1-0716-1441-9_2. Methods Mol Biol. 2021. PMID: 34228282
-
Accurate de novo design of heterochiral protein-protein interactions.Cell Res. 2024 Dec;34(12):846-858. doi: 10.1038/s41422-024-01014-2. Epub 2024 Aug 14. Cell Res. 2024. PMID: 39143121 Free PMC article.
-
Phosphoregulated orthogonal signal transduction in mammalian cells.Nat Commun. 2020 Jun 18;11(1):3085. doi: 10.1038/s41467-020-16895-1. Nat Commun. 2020. PMID: 32555187 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

