Subtropical hibernation in juvenile tegu lizards (Salvator merianae): insights from intestine redox dynamics
- PMID: 29921981
- PMCID: PMC6008456
- DOI: 10.1038/s41598-018-27263-x
Subtropical hibernation in juvenile tegu lizards (Salvator merianae): insights from intestine redox dynamics
Abstract
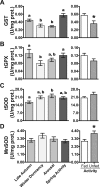
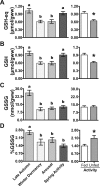
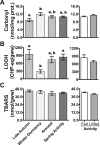
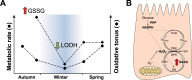
Juvenile tegu lizards (Salvator merianae) experience gradual and mild temperature changes from autumn to winter in their habitat. This tropical/subtropical reptile enter a state of dormancy, with an 80% reduction in metabolic rate, that remains almost constant during winter. The redox metabolism in non-mammalian vertebrates that hibernate under such distinguished conditions is poorly understood. We analyzed the redox metabolism in the intestine of juvenile tegus during different stages of their first annual cycle. The effect of food deprivation (in spring) was also studied to compare with fasting during hibernation. Both winter dormancy and food deprivation caused decreases in reduced glutathione levels and glutathione transferase activity. While glutathione peroxidase and glutathione transferase activities decreased during winter dormancy, as well as glutathione (GSH) levels, other antioxidant enzymes (catalase, superoxide dismutase and glutathione reductase) remained unchanged. Notably, levels of disulfide glutathione (GSSG) were 2.1-fold higher in late autumn, when animals were in the process of depressing metabolism towards hibernation. This increased "oxidative tonus" could be due to a disruption in NADPH-dependent antioxidant systems. In dormancy, GSSG and lipid hydroperoxides were diminished by 60-70%. The results suggest that the entrance into hibernation is the main challenge for the redox homeostasis in the intestine of juvenile tegus.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The consequences of seasonal fasting during the dormancy of tegu lizards (Salvator merianae) on their postprandial metabolic response.J Exp Biol. 2018 Apr 19;221(Pt 8):jeb176156. doi: 10.1242/jeb.176156. J Exp Biol. 2018. PMID: 29530973
-
Hibernation induces glutathione redox imbalance in ground squirrel intestine.J Comp Physiol B. 2003 Jun;173(4):269-76. doi: 10.1007/s00360-003-0330-3. Epub 2003 Mar 7. J Comp Physiol B. 2003. PMID: 12820005
-
Morphological and metabolic adjustments in the small intestine to energy demands of growth, storage, and fasting in the first annual cycle of a hibernating lizard (Tupinambis merianae).Comp Biochem Physiol A Mol Integr Physiol. 2016 May;195:55-64. doi: 10.1016/j.cbpa.2016.02.002. Epub 2016 Feb 9. Comp Biochem Physiol A Mol Integr Physiol. 2016. PMID: 26872995
-
Glutathione status and antioxidant enzymes in a crocodilian species from the swamps of the Brazilian Pantanal.Comp Biochem Physiol A Mol Integr Physiol. 2012 Oct;163(2):189-98. doi: 10.1016/j.cbpa.2012.06.006. Epub 2012 Jun 26. Comp Biochem Physiol A Mol Integr Physiol. 2012. PMID: 22750313
-
Intestinal glutathione: determinant of mucosal peroxide transport, metabolism, and oxidative susceptibility.Toxicol Appl Pharmacol. 2005 May 1;204(3):320-8. doi: 10.1016/j.taap.2004.11.016. Toxicol Appl Pharmacol. 2005. PMID: 15845421 Review.
Cited by
-
Dynamics of Redox Metabolism during Complete Metamorphosis of Insects: Insights from the Sunflower Caterpillar Chlosyne lacinia (Lepidoptera).Antioxidants (Basel). 2024 Aug 7;13(8):959. doi: 10.3390/antiox13080959. Antioxidants (Basel). 2024. PMID: 39199204 Free PMC article.
-
Variations in oxidative stress and antioxidant defense level during different phases of hibernation in common Asian toad, Duttaphrynus melanostictus.Biol Open. 2021 Jul 15;10(7):bio058567. doi: 10.1242/bio.058567. Epub 2021 Aug 5. Biol Open. 2021. PMID: 34350459 Free PMC article.
-
Carry-Over Effects of Desiccation Stress on the Oxidative Status of Fasting Anuran Juveniles.Front Physiol. 2021 Dec 2;12:783288. doi: 10.3389/fphys.2021.783288. eCollection 2021. Front Physiol. 2021. PMID: 34925072 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

