A Quantitative Tractography Study Into the Connectivity, Segmentation and Laterality of the Human Inferior Longitudinal Fasciculus
- PMID: 29922132
- PMCID: PMC5996125
- DOI: 10.3389/fnana.2018.00047
A Quantitative Tractography Study Into the Connectivity, Segmentation and Laterality of the Human Inferior Longitudinal Fasciculus
Abstract
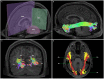
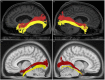
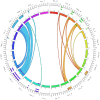
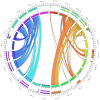
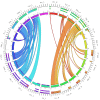
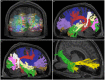
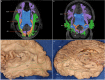
The human inferior longitudinal fasciculus (ILF) is a ventral, temporo-occipital association tract. Though described in early neuroanatomical works, its existence was later questioned. Application of in vivo tractography to the neuroanatomical study of the ILF has generally confirmed its existence, however, consensus is lacking regarding its subdivision, laterality and connectivity. Further, there is a paucity of detailed neuroanatomic data pertaining to the exact anatomy of the ILF. Generalized Q-Sampling imaging (GQI) is a non-tensor tractographic modality permitting high resolution imaging of white-matter structures. As it is a non-tensor modality, it permits visualization of crossing fibers and accurate delineation of close-proximity fiber-systems. We applied deterministic GQI tractography to data from 30 healthy subjects and a large-volume, averaged diffusion atlas, to delineate ILF anatomy. Post-mortem white matter dissection was also carried out in three cadaveric specimens for further validation. The ILF was found in all 60 hemispheres. At its occipital extremity, ILF fascicles demonstrated a bifurcated, ventral-dorsal morphological termination pattern, which we used to further subdivide the bundle for detailed analysis. These divisions were consistent across the subject set and within the atlas. We applied quantitative techniques to study connectivity strength of the ILF at its anterior and posterior extremities. Overall, both morphological divisions, and the un-separated ILF, demonstrated strong leftward-lateralized connectivity patterns. Leftward-lateralization was also found for ILF volumes across the subject set. Due to connective and volumetric leftward-dominance and ventral location, we postulate the ILFs role in the semantic system. Further, our results are in agreement with functional and lesion-based postulations pertaining to the ILFs role in facial recognition.
Keywords: inferior longitudinal fasciculus; non-tensor tractography; semantic anatomy; tractography; white matter anatomy.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

