Deciphering the Astrocyte Reaction in Alzheimer's Disease
- PMID: 29922147
- PMCID: PMC5996928
- DOI: 10.3389/fnagi.2018.00114
Deciphering the Astrocyte Reaction in Alzheimer's Disease
Abstract
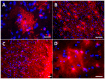
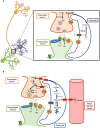
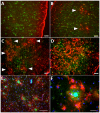
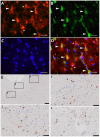
Reactive astrocytes were identified as a component of senile amyloid plaques in the cortex of Alzheimer's disease (AD) patients several decades ago. However, their role in AD pathophysiology has remained elusive ever since, in part owing to the extrapolation of the literature from primary astrocyte cultures and acute brain injury models to a chronic neurodegenerative scenario. Recent accumulating evidence supports the idea that reactive astrocytes in AD acquire neurotoxic properties, likely due to both a gain of toxic function and a loss of their neurotrophic effects. However, the diversity and complexity of this glial cell is only beginning to be unveiled, anticipating that astrocyte reaction might be heterogeneous as well. Herein we review the evidence from mouse models of AD and human neuropathological studies and attempt to decipher the main conundrums that astrocytes pose to our understanding of AD development and progression. We discuss the morphological features that characterize astrocyte reaction in the AD brain, the consequences of astrocyte reaction for both astrocyte biology and AD pathological hallmarks, and the molecular pathways that have been implicated in this reaction.
Keywords: Alzheimer’s disease; amyloid plaques; astrocytes; glia; microglia; neurofibrillary tangles.
Figures
References
-
- Akiyama H., Mori H., Saido T., Kondo H., Ikeda K., McGeer P. L. (1999). Occurrence of the diffuse amyloid β-protein (Aβ) deposits with numerous Aβ-containing glial cells in the cerebral cortex of patients with Alzheimer’s disease. Glia 25, 324–331. 10.1002/(sici)1098-1136(19990215)25:4<324::aid-glia2>3.0.co;2-5 - DOI - PubMed
-
- Alcolea D., Vilaplana E., Pegueroles J., Montal V., Sánchez-Juan P., González-Suárez A., et al. (2015). Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol. Aging 36, 2018–2023. 10.1016/j.neurobiolaging.2015.03.001 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

