The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer's Disease
- PMID: 29922148
- PMCID: PMC5996906
- DOI: 10.3389/fnagi.2018.00118
The Physiological Roles of Amyloid-β Peptide Hint at New Ways to Treat Alzheimer's Disease
Abstract
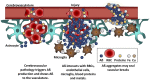
Amyloid-ß (Aß) is best known as the misfolded peptide that is involved in the pathogenesis of Alzheimer's disease (AD), and it is currently the primary therapeutic target in attempts to arrest the course of this disease. This notoriety has overshadowed evidence that Aß serves several important physiological functions. Aß is present throughout the lifespan, it has been found in all vertebrates examined thus far, and its molecular sequence shows a high degree of conservation. These features are typical of a factor that contributes significantly to biological fitness, and this suggestion has been supported by evidence of functions that are beneficial for the brain. The putative roles of Aß include protecting the body from infections, repairing leaks in the blood-brain barrier, promoting recovery from injury, and regulating synaptic function. Evidence for these beneficial roles comes from in vitro and in vivo studies, which have shown that the cellular production of Aß rapidly increases in response to a physiological challenge and often diminishes upon recovery. These roles are further supported by the adverse outcomes of clinical trials that have attempted to deplete Aß in order to treat AD. We suggest that anti-Aß therapies will produce fewer adverse effects if the known triggers of Aß deposition (e.g., pathogens, hypertension, and diabetes) are addressed first.
Keywords: ARIA; antimicrobial; cancer; cerebrovascular; immune system; infection; seizure; traumatic injury.
Figures

References
-
- Abramowski D., Wiederhold K.-H., Furrer U., Jaton A.-L., Neuenschwander A., Runser M.-J., et al. (2008). Dynamics of Abeta turnover and deposition in different beta-amyloid precursor protein transgenic mouse models following gamma-secretase inhibition. J. Pharmacol. Exp. Ther. 327, 411–424. 10.1124/jpet.108.140327 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

