Vesicle-Mediated Control of Cell Function: The Role of Extracellular Matrix and Microenvironment
- PMID: 29922170
- PMCID: PMC5996101
- DOI: 10.3389/fphys.2018.00651
Vesicle-Mediated Control of Cell Function: The Role of Extracellular Matrix and Microenvironment
Abstract
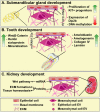
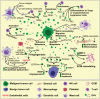
Extracellular vesicles (EVs) - including exosomes, microvesicles and apoptotic bodies - have received much scientific attention last decade as mediators of a newly discovered cell-to-cell communication system, acting at short and long distances. EVs carry biologically active molecules, thus providing signals that influence a spectrum of functions in recipient cells during various physiological and pathological processes. Recent findings point to EVs as very attractive immunomodulatory therapeutic agents, vehicles for drug delivery and diagnostic and prognostic biomarkers in liquid biopsies. In addition, EVs interact with and regulate the synthesis of extracellular matrix (ECM) components, which is crucial for organ development and wound healing, as well as bone and cardiovascular calcification. EVs carrying matrix metalloproteinases (MMPs) are involved in ECM remodeling, thus modifying tumor microenvironment and contributing to premetastatic niche formation and angiogenesis. Here we review the role of EVs in control of cell function, with emphasis on their interaction with ECM and microenvironment in health and disease.
Keywords: cell function; exosomes; extracellular matrix; extracellular vesicles; microenvironment.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

