Prospects for Biocontrol of Vibrio parahaemolyticus Contamination in Blue Mussels (Mytilus edulus)-A Year-Long Study
- PMID: 29922246
- PMCID: PMC5996151
- DOI: 10.3389/fmicb.2018.01043
Prospects for Biocontrol of Vibrio parahaemolyticus Contamination in Blue Mussels (Mytilus edulus)-A Year-Long Study
Abstract
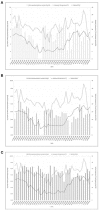
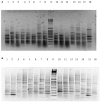
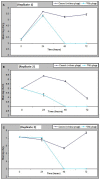
Vibrio parahaemolyticus is an environmental organism normally found in subtropical estuarine environments which can cause seafood-related human infections. Clinical disease is associated with diagnostic presence of tdh and/or trh virulence genes and identification of these genes in our preliminary isolates from retail shellfish prompted a year-long surveillance of isolates from a temperate estuary in the north of England. The microbial and environmental analysis of 117 samples of mussels, seawater or sediment showed the presence of V. parahaemolyticus from mussels (100%) at all time-points throughout the year including the colder months although they were only recovered from 94.9% of seawater and 92.3% of sediment samples. Throughout the surveillance, 96 isolates were subjected to specific PCR for virulence genes and none tested positive for either. The common understanding that consuming poorly cooked mussels only represents a risk of infection during summer vacations therefore is challenged. Further investigations with V. parahaemolyticus using RAPD-PCR cluster analysis showed a genetically diverse population. There was no distinct clustering for "environmental" or "clinical" reference strains although a wide variability and heterogeneity agreed with other reports. Continued surveillance of isolates to allay public health risks are justified since geographical distribution and composition of V. parahaemolyticus varies with Future Ocean warming and the potential of environmental strains to acquire virulence genes from pathogenic isolates. The prospects for intervention by phage-mediated biocontrol to reduce or eradicate V. parahaemolyticus in mussels was also investigated. Bacteriophages isolated from enriched samples collected from the river Humber were assessed for their ability to inhibit the growth of V. parahaemolyticus strains in-vitro and in-vivo (with live mussels). V. parahaemolyticus were significantly reduced in-vitro, by an average of 1 log-2 log units and in-vivo, significant reduction of the organisms in mussels occurred in three replicate experimental tank set ups with a "phage cocktail" containing 12 different phages. Our perspective biocontrol study suggests that a cocktail of specific phages targeted against strains of V. parahaemolyticus provides good evidence in an experimental setting of the valuable potential of phage as a decontamination agent in natural or industrial mussel processing (343w).
Keywords: Mytilus edulis; RAPD-PCR; Vibrio parahaemolyticus; bacteriophage; chromogenic agar; genetic diversity; temperate estuary.
Figures




Similar articles
-
Longitudinal Study of Total and Pathogenic Vibrio parahaemolyticus (tdh+ and/or trh+) in Two Natural Extraction Areas of Mytilus chilensis in Southern Chile.Front Microbiol. 2021 Mar 18;12:621737. doi: 10.3389/fmicb.2021.621737. eCollection 2021. Front Microbiol. 2021. PMID: 33815309 Free PMC article.
-
Prevalence, Virulence Characterization, AMR Pattern and Genetic Relatedness of Vibrio parahaemolyticus Isolates From Retail Seafood of Kerala, India.Front Microbiol. 2020 Apr 7;11:592. doi: 10.3389/fmicb.2020.00592. eCollection 2020. Front Microbiol. 2020. PMID: 32318050 Free PMC article.
-
Trh (tdh-/trh+) gene analysis of clinical, environmental and food isolates of Vibrio parahaemolyticus as a tool for investigating pathogenicity.Int J Food Microbiol. 2016 May 16;225:43-53. doi: 10.1016/j.ijfoodmicro.2016.02.016. Epub 2016 Mar 2. Int J Food Microbiol. 2016. PMID: 26990408
-
Roles of thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) in Vibrio parahaemolyticus.Front Microbiol. 2015 Jan 22;5:805. doi: 10.3389/fmicb.2014.00805. eCollection 2014. Front Microbiol. 2015. PMID: 25657643 Free PMC article. Review.
-
Ecological fitness and virulence features of Vibrio parahaemolyticus in estuarine environments.Appl Microbiol Biotechnol. 2017 Mar;101(5):1781-1794. doi: 10.1007/s00253-017-8096-9. Epub 2017 Jan 31. Appl Microbiol Biotechnol. 2017. PMID: 28144705 Review.
Cited by
-
Marine Bacteriophages as Next-Generation Therapeutics: Insights into Antimicrobial Potential and Application.Viruses. 2025 Jul 10;17(7):971. doi: 10.3390/v17070971. Viruses. 2025. PMID: 40733588 Free PMC article. Review.
-
Phage Therapy as a Focused Management Strategy in Aquaculture.Int J Mol Sci. 2021 Sep 28;22(19):10436. doi: 10.3390/ijms221910436. Int J Mol Sci. 2021. PMID: 34638776 Free PMC article. Review.
-
A Concise Overview of Studies on Successful Real-World Applications of Bacteriophages in Aquaculture.Viruses. 2024 Nov 28;16(12):1843. doi: 10.3390/v16121843. Viruses. 2024. PMID: 39772153 Free PMC article. Review.
-
Phages as a Cohesive Prophylactic and Therapeutic Approach in Aquaculture Systems.Antibiotics (Basel). 2020 Sep 1;9(9):564. doi: 10.3390/antibiotics9090564. Antibiotics (Basel). 2020. PMID: 32882880 Free PMC article. Review.
-
Role of Bacteriophages in the Evolution of Pathogenic Vibrios and Lessons for Phage Therapy.Adv Exp Med Biol. 2023;1404:149-173. doi: 10.1007/978-3-031-22997-8_8. Adv Exp Med Biol. 2023. PMID: 36792875 Free PMC article.
References
-
- Adams M. H. (1959). Bacteriophages. New York, NY: Interscience.
-
- Alonso M. D., Rodriguez J., Borrego J. J. (2002). Characterization of marine bacteriophages isolated from Alboran sea (Western Mediterranean). J. Plankton Res. 24, 1079–1087. 10.1093/plankt/24.10.1079 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

