Virulence Gene Sequencing Highlights Similarities and Differences in Sequences in Listeria monocytogenes Serotype 1/2a and 4b Strains of Clinical and Food Origin From 3 Different Geographic Locations
- PMID: 29922249
- PMCID: PMC5996115
- DOI: 10.3389/fmicb.2018.01103
Virulence Gene Sequencing Highlights Similarities and Differences in Sequences in Listeria monocytogenes Serotype 1/2a and 4b Strains of Clinical and Food Origin From 3 Different Geographic Locations
Abstract
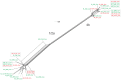
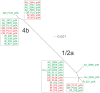
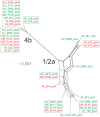
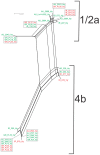
The prfA-virulence gene cluster (pVGC) is the main pathogenicity island in Listeria monocytogenes, comprising the prfA, plcA, hly, mpl, actA, and plcB genes. In this study, the pVGC of 36 L. monocytogenes isolates with respect to different serotypes (1/2a or 4b), geographical origin (Australia, Greece or Ireland) and isolation source (food-associated or clinical) was characterized. The most conserved genes were prfA and hly, with the lowest nucleotide diversity (π) among all genes (P < 0.05), and the lowest number of alleles, substitutions and non-synonymous substitutions for prfA. Conversely, the most diverse gene was actA, which presented the highest number of alleles (n = 20) and showed the highest nucleotide diversity. Grouping by serotype had a significantly lower π value (P < 0.0001) compared to isolation source or geographical origin, suggesting a distinct and well-defined unit compared to other groupings. Among all tested genes, only hly and mpl were those with lower nucleotide diversity in 1/2a serotype than 4b serotype, reflecting a high within-1/2a serotype divergence compared to 4b serotype. Geographical divergence was noted with respect to the hly gene, where serotype 4b Irish strains were distinct from Greek and Australian strains. Australian strains showed less diversity in plcB and mpl relative to Irish or Greek strains. Notable differences regarding sequence mutations were identified between food-associated and clinical isolates in prfA, actA, and plcB sequences. Overall, these results indicate that virulence genes follow different evolutionary pathways, which are affected by a strain's origin and serotype and may influence virulence and/or epidemiological dominance of certain subgroups.
Keywords: Listeria monocytogenes; actA; diversity; gene sequencing; hly; prfA; virulence.
Figures




References
-
- Brosch R., Catimel B., Milon G., Buchrieser C., Vindel E., Rocourt J. (1993). Virulence heterogeneity of Listeria monocytogenes strains from various sources (food, human, animal) in immunocompetent mice and its association with typing characteristics. J. Food Prot. 56, 296–301. 10.4315/0362-028X-56.4.297 - DOI - PubMed
-
- Bueno V. F., Banerjee P., Banada P. P., José De Mesquita A., Lemes-Marques E. G., Bhunia A. K. (2010). Characterization of Listeria monocytogenes isolates of food and human origins from Brazil using molecular typing procedures and in vitro cell culture assays. Int. J. Environ. Health Res. 20, 43–59. 10.1080/09603120903281283 - DOI - PubMed
-
- Chakraborty T., Ebel F., Wehland J., Dufrenne J., Notermans S. (1994). Naturally occurring virulence-attenuated isolates of Listeria monocytogenes capable of inducing long term protection against infection by virulent strains of homologous and heterologous serotypes. FEMS Immunol. Med. Microbiol. 10, 1–9. 10.1111/j.1574-695X.1994.tb00004.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

