Co-administered Tag-Less Toxoid Fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (Multiepitope Fusion Antigen) Induce Neutralizing Antibodies to 7 Adhesins (CFA/I, CS1-CS6) and Both Enterotoxins (LT, STa) of Enterotoxigenic Escherichia coli (ETEC)
- PMID: 29922268
- PMCID: PMC5996201
- DOI: 10.3389/fmicb.2018.01198
Co-administered Tag-Less Toxoid Fusion 3xSTaN12S-mnLTR192G/L211A and CFA/I/II/IV MEFA (Multiepitope Fusion Antigen) Induce Neutralizing Antibodies to 7 Adhesins (CFA/I, CS1-CS6) and Both Enterotoxins (LT, STa) of Enterotoxigenic Escherichia coli (ETEC)
Abstract
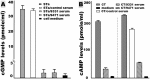
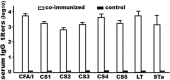
Enterotoxigenic Escherichia coli (ETEC) bacteria remain a leading cause of children's diarrhea and travelers' diarrhea. Vaccines that induce antibodies to block ETEC bacterial adherence and to neutralize toxin enterotoxicity can be effective against ETEC-associated diarrhea. Recent studies showed that 6xHis-tagged CFA/I/II/IV multiepitope fusion antigen (MEFA) induced broad-spectrum antibodies to inhibit adherence of the seven most important ETEC adhesins (CFA/I, CS1 to CS6) (Ruan et al., 2014a) and 6xHis-tagged toxoid fusion antigen 3xSTaN12S-mnLTR192G/L211A (previously named as 3xSTaN12S-dmLT) elicited antibodies to neutralize both heat-labile toxin (LT) and heat-stable toxin (STa) produced by ETEC strains (Ruan et al., 2014b). In this study, we constructed two new genes to express tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and tag-less CFA/I/II/IV MEFA and then examined immunogenicity of each tag-less protein in mouse immunization. We further combined two tag-less proteins and investigated antigen co-administration in mice. Data showed that mice immunized with tag-less 3xSTaN12S-mnLTR192G/L211A or tag-less CFA/I/II/IV MEFA developed antigen-specific IgG antibody responses, and mice co-administered with two tag-less proteins induced neutralizing antibodies against seven adhesins and both toxins. These results indicated tag-less toxoid fusion 3xSTaN12S-mnLTR192G/L211A and tag-less CFA/I/II/IV MEFA administered individually or combined induced neutralizing antitoxin and/or anti-adhesin antibodies, and suggested the potential application of two tag-less proteins for ETEC vaccine development.
Keywords: ETEC (enterotoxigenic Escherichia coli); MEFA (multiepitope fusion antigen); antibody; diarrhea; toxoid fusion; vaccine.
Figures



Similar articles
-
Enterotoxigenic Escherichia coli Adhesin-Toxoid Multiepitope Fusion Antigen CFA/I/II/IV-3xSTaN12S-mnLTG192G/L211A-Derived Antibodies Inhibit Adherence of Seven Adhesins, Neutralize Enterotoxicity of LT and STa Toxins, and Protect Piglets against Diarrhea.Infect Immun. 2018 Feb 20;86(3):e00550-17. doi: 10.1128/IAI.00550-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29263112 Free PMC article.
-
Intradermally Administered Enterotoxigenic Escherichia coli Vaccine Candidate MecVax Induces Functional Serum Immunoglobulin G Antibodies against Seven Adhesins (CFA/I and CS1 through CS6) and Both Toxins (STa and LT).Appl Environ Microbiol. 2022 Feb 22;88(4):e0213921. doi: 10.1128/AEM.02139-21. Epub 2021 Dec 22. Appl Environ Microbiol. 2022. PMID: 34936832 Free PMC article.
-
Genetic fusions of a CFA/I/II/IV MEFA (multiepitope fusion antigen) and a toxoid fusion of heat-stable toxin (STa) and heat-labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC) retain broad anti-CFA and antitoxin antigenicity.PLoS One. 2015 Mar 24;10(3):e0121623. doi: 10.1371/journal.pone.0121623. eCollection 2015. PLoS One. 2015. PMID: 25803825 Free PMC article.
-
Current Progress in Developing Subunit Vaccines against Enterotoxigenic Escherichia coli-Associated Diarrhea.Clin Vaccine Immunol. 2015 Sep;22(9):983-91. doi: 10.1128/CVI.00224-15. Epub 2015 Jul 1. Clin Vaccine Immunol. 2015. PMID: 26135975 Free PMC article. Review.
-
Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals.Microbiologia. 1991 Sep;7(2):57-73. Microbiologia. 1991. PMID: 1684712 Review.
Cited by
-
A polyvalent multiepitope protein cross-protects against Vibrio cholerae infection in rabbit colonization and passive protection models.Proc Natl Acad Sci U S A. 2022 Dec 13;119(50):e2202938119. doi: 10.1073/pnas.2202938119. Epub 2022 Dec 5. Proc Natl Acad Sci U S A. 2022. PMID: 36469767 Free PMC article.
-
Adjuvant effect of enterotoxigenic Escherichia coli (ETEC) double-mutant heat-labile toxin (dmLT) on systemic immunogenicity induced by the CFA/I/II/IV MEFA ETEC vaccine: Dose-related enhancement of antibody responses to seven ETEC adhesins (CFA/I, CS1-CS6).Hum Vaccin Immunother. 2020;16(2):419-425. doi: 10.1080/21645515.2019.1649555. Epub 2019 Aug 23. Hum Vaccin Immunother. 2020. PMID: 31361177 Free PMC article.
-
Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells.Front Cell Infect Microbiol. 2019 Aug 21;9:299. doi: 10.3389/fcimb.2019.00299. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31497538 Free PMC article. Review.
-
Immunogenicity and preclinical efficacy characterization of ShecVax, a combined vaccine against Shigella and enterotoxigenic Escherichia coli.Infect Immun. 2025 Jun 10;93(6):e0000425. doi: 10.1128/iai.00004-25. Epub 2025 Apr 10. Infect Immun. 2025. PMID: 40208039 Free PMC article.
-
Vaccines against gastroenteritis, current progress and challenges.Gut Microbes. 2020 Nov 1;11(6):1486-1517. doi: 10.1080/19490976.2020.1770666. Epub 2020 Jun 18. Gut Microbes. 2020. PMID: 32552414 Free PMC article. Review.
References
-
- Bölin I., Wiklund G., Qadri F., Torres O., Bourgeois A. L., Savarino S., et al. . (2006). Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. J. Clin. Microbiol. 44, 3872–3877. 10.1128/JCM.00790-06 - DOI - PMC - PubMed
-
- Duan Q., Huang J., Xiao N., Seo H., Zhang W. (2017a). Neutralizing anti-STa antibodies derived from enterotoxigenic Escherichia coli (ETEC) toxoid fusions with heat-stable toxin (STa) mutant STaN12S, STaL9A/N12S or STaN12S/A14T show little cross-reactivity with guanylin or uroguanylin. Appl. Environ. Microbiol. 84. 10.1128/AEM.01737-17 - DOI - PMC - PubMed
-
- Duan Q., Lee K. H., Nandre R. M., Garcia C., Chen J., Zhang W. (2017b). MEFA (multiepitope fusion antigen)-novel technology for structural vaccinology, proof from computational and empirical immunogenicity characterization of an enterotoxigenic Escherichia coli (ETEC) Adhesin MEFA. J. Vaccines Vaccin. 8. 10.4172/2157-7560.1000367 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

