A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge
- PMID: 29922441
- PMCID: PMC5987690
- DOI: 10.1302/2046-3758.73.BJR-2017-0270.R1
A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge
Abstract
Despite its intrinsic ability to regenerate form and function after injury, bone tissue can be challenged by a multitude of pathological conditions. While innovative approaches have helped to unravel the cascades of bone healing, this knowledge has so far not improved the clinical outcomes of bone defect treatment. Recent findings have allowed us to gain in-depth knowledge about the physiological conditions and biological principles of bone regeneration. Now it is time to transfer the lessons learned from bone healing to the challenging scenarios in defects and employ innovative technologies to enable biomaterial-based strategies for bone defect healing. This review aims to provide an overview on endogenous cascades of bone material formation and how these are transferred to new perspectives in biomaterial-driven approaches in bone regeneration. Cite this article: T. Winkler, F. A. Sass, G. N. Duda, K. Schmidt-Bleek. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Joint Res 2018;7:232-243. DOI: 10.1302/2046-3758.73.BJR-2017-0270.R1.
Keywords: Biomaterials; Bone; Fracture; Healing phases; Mineralization; Scaffold.
Conflict of interest statement
Conflicts of Interest Statement: None declared
Figures


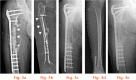


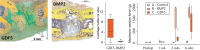

References
-
- Haas NP. Callus modulation–fiction or reality? Chirurg 2000;71:987-988. - PubMed
-
- Willie BPA, Schmidt-Bleek K, Cipitria A, et al. Designing biomimetic scaffolds for bone regeneration: why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter 2010;6:4976.
-
- Killington K, Mafi R, Mafi P, Khan W. A systematic review of clinical studies investigating mesenchymal stem cells for fracture non-union and bone defects. Curr Stem Cell Res Ther 2017. (Epub ahead of print) PMID: 28914208. - PubMed
-
- Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 2013;8:136. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

