Harnessing extracellular vesicles to direct endochondral repair of large bone defects
- PMID: 29922444
- PMCID: PMC5987693
- DOI: 10.1302/2046-3758.74.BJR-2018-0006
Harnessing extracellular vesicles to direct endochondral repair of large bone defects
Abstract
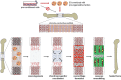
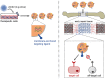
Large bone defects remain a tremendous clinical challenge. There is growing evidence in support of treatment strategies that direct defect repair through an endochondral route, involving a cartilage intermediate. While culture-expanded stem/progenitor cells are being evaluated for this purpose, these cells would compete with endogenous repair cells for limited oxygen and nutrients within ischaemic defects. Alternatively, it may be possible to employ extracellular vesicles (EVs) secreted by culture-expanded cells for overcoming key bottlenecks to endochondral repair, such as defect vascularization, chondrogenesis, and osseous remodelling. While mesenchymal stromal/stem cells are a promising source of therapeutic EVs, other donor cells should also be considered. The efficacy of an EV-based therapeutic will likely depend on the design of companion scaffolds for controlled delivery to specific target cells. Ultimately, the knowledge gained from studies of EVs could one day inform the long-term development of synthetic, engineered nanovesicles. In the meantime, EVs harnessed from in vitro cell culture have near-term promise for use in bone regenerative medicine. This narrative review presents a rationale for using EVs to improve the repair of large bone defects, highlights promising cell sources and likely therapeutic targets for directing repair through an endochondral pathway, and discusses current barriers to clinical translation. Cite this article: E. Ferreira, R. M. Porter. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res 2018;7:263-273. DOI: 10.1302/2046-3758.74.BJR-2018-0006.
Keywords: Critical-sized bone defects; Endochondral ossification; Extracellular vesicles; Mesenchymal stromal cells.
Conflict of interest statement
Conflicts of Interest Statement: None declared.
Figures



Similar articles
-
An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo.Tissue Eng Part A. 2016 Mar;22(5-6):556-67. doi: 10.1089/ten.TEA.2015.0457. Tissue Eng Part A. 2016. PMID: 26896424
-
3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects.Acta Biomater. 2020 Sep 1;113:130-143. doi: 10.1016/j.actbio.2020.05.040. Epub 2020 Jun 4. Acta Biomater. 2020. PMID: 32505800
-
3D printed microchannel networks to direct vascularisation during endochondral bone repair.Biomaterials. 2018 Apr;162:34-46. doi: 10.1016/j.biomaterials.2018.01.057. Epub 2018 Feb 1. Biomaterials. 2018. PMID: 29432987
-
Extracellular vesicle-loaded hydrogels for tissue repair and regeneration.Mater Today Bio. 2022 Dec 21;18:100522. doi: 10.1016/j.mtbio.2022.100522. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36593913 Free PMC article. Review.
-
Recapitulating endochondral ossification: a promising route to in vivo bone regeneration.J Tissue Eng Regen Med. 2015 Aug;9(8):889-902. doi: 10.1002/term.1918. Epub 2014 Jun 11. J Tissue Eng Regen Med. 2015. PMID: 24916192 Review.
Cited by
-
Extracellular Vesicles from Mesenchymal Stem Cells as Novel Treatments for Musculoskeletal Diseases.Cells. 2019 Dec 31;9(1):98. doi: 10.3390/cells9010098. Cells. 2019. PMID: 31906087 Free PMC article. Review.
-
Extracellular Vesicles in Premature Aging and Diseases in Adulthood Due to Developmental Exposures.Aging Dis. 2021 Sep 1;12(6):1516-1535. doi: 10.14336/AD.2021.0322. eCollection 2021 Sep. Aging Dis. 2021. PMID: 34527425 Free PMC article. Review.
-
Bioengineering extracellular vesicles: smart nanomaterials for bone regeneration.J Nanobiotechnology. 2023 Apr 27;21(1):137. doi: 10.1186/s12951-023-01895-2. J Nanobiotechnology. 2023. PMID: 37106449 Free PMC article. Review.
-
Exploring a Chemotactic Role for EVs from Progenitor Cell Populations of Human Exfoliated Deciduous Teeth for Promoting Migration of Naïve BMSCs in Bone Repair Process.Stem Cells Int. 2021 Mar 17;2021:6681771. doi: 10.1155/2021/6681771. eCollection 2021. Stem Cells Int. 2021. PMID: 33815511 Free PMC article.
-
Extracellular vesicles derived from mesenchymal stem cells containing microRNA-381 protect against spinal cord injury in a rat model via the BRD4/WNT5A axis.Bone Joint Res. 2021 May;10(5):328-339. doi: 10.1302/2046-3758.105.BJR-2020-0020.R1. Bone Joint Res. 2021. PMID: 34024119 Free PMC article.
References
-
- Stevenson S. Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop Relat Res 1998;355S(suppl):S239-S246. - PubMed
-
- Levin LS. Vascularized fibula graft for the traumatically induced long-bone defect. Journal Am Academy Ortho Surgeons 2006;14:S175-176. - PubMed
-
- Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 2010;41:27-37. - PubMed
-
- Mauffrey C, Barlow BT, Smith W. Management of segmental bonedefects. J Am Acad Orthop Surg 2015;23:143-153. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

