Melanoma treatment in review
- PMID: 29922629
- PMCID: PMC5995433
- DOI: 10.2147/ITT.S134842
Melanoma treatment in review
Abstract
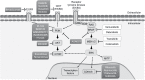
Melanoma represents the most aggressive and the deadliest form of skin cancer. Current therapeutic approaches include surgical resection, chemotherapy, photodynamic therapy, immunotherapy, biochemotherapy, and targeted therapy. The therapeutic strategy can include single agents or combined therapies, depending on the patient's health, stage, and location of the tumor. The efficiency of these treatments can be decreased due to the development of diverse resistance mechanisms. New therapeutic targets have emerged from studies of the genetic profile of melanocytes and from the identification of molecular factors involved in the pathogenesis of the malignant transformation. In this review, we aim to survey therapies approved and under evaluation for melanoma treatment and relevant research on the molecular mechanisms underlying melanomagenesis.
Keywords: cancer; melanoma; targets; therapy.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


References
-
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. - PubMed
-
- Davids L, Kleemann B. The menace of melanoma: a photodynamic approach to adjunctive cancer therapy. In: Huynh Thien Duc G, editor. Melanoma – From Early Detection to Treatment. Croatia: INTECH Open Access Publisher; 2013.
-
- Tolleson WH. Human melanocyte biology, toxicology, and pathology. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2005;23(2):105–161. - PubMed
-
- Pópulo H, Soares P, Lopes JM. Insights into melanoma: targeting the mTOR pathway for therapeutics. Expert Opin Ther Targets. 2012;16(7):689–705. - PubMed
-
- American Cancer Society Cancer Facts & Figures 2017. 2017. [Accessed November 2017]. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/a....
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

