Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis
- PMID: 29922644
- PMCID: PMC5996923
- DOI: 10.3389/fchem.2018.00135
Mechanisms of Aquaporin-Facilitated Cancer Invasion and Metastasis
Abstract
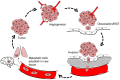
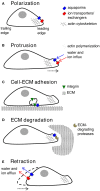
Cancer is a leading cause of death worldwide, and its incidence is rising with numbers expected to increase 70% in the next two decades. The fact that current mainline treatments for cancer patients are accompanied by debilitating side effects prompts a growing demand for new therapies that not only inhibit growth and proliferation of cancer cells, but also control invasion and metastasis. One class of targets gaining international attention is the aquaporins, a family of membrane-spanning water channels with diverse physiological functions and extensive tissue-specific distributions in humans. Aquaporins-1,-2,-3,-4,-5,-8, and-9 have been linked to roles in cancer invasion, and metastasis, but their mechanisms of action remain to be fully defined. Aquaporins are implicated in the metastatic cascade in processes of angiogenesis, cellular dissociation, migration, and invasion. Cancer invasion and metastasis are proposed to be potentiated by aquaporins in boosting tumor angiogenesis, enhancing cell volume regulation, regulating cell-cell and cell-matrix adhesions, interacting with actin cytoskeleton, regulating proteases and extracellular-matrix degrading molecules, contributing to the regulation of epithelial-mesenchymal transitions, and interacting with signaling pathways enabling motility and invasion. Pharmacological modulators of aquaporin channels are being identified and tested for therapeutic potential, including compounds derived from loop diuretics, metal-containing organic compounds, plant natural products, and other small molecules. Further studies on aquaporin-dependent functions in cancer metastasis are needed to define the differential contributions of different classes of aquaporin channels to regulation of fluid balance, cell volume, small solute transport, signal transduction, their possible relevance as rate limiting steps, and potential values as therapeutic targets for invasion and metastasis.
Keywords: aquaporin; cancer; cell migration; drug; invasion; metastasis; pharmacology.
Figures
References
-
- Aggarwal B. B., Kumar A., Bharti A. C. (2003). Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 23, 363–398. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

