First-in-human randomized clinical trials of the safety and efficacy of tanezumab for treatment of chronic knee osteoarthritis pain or acute bunionectomy pain
- PMID: 29922745
- PMCID: PMC5999411
- DOI: 10.1097/PR9.0000000000000653
First-in-human randomized clinical trials of the safety and efficacy of tanezumab for treatment of chronic knee osteoarthritis pain or acute bunionectomy pain
Abstract
Introduction: The neurotrophin nerve growth factor has a demonstrated role in pain transduction and pathophysiology.
Objectives: Two randomized, double-blind, placebo-controlled, phase 1 studies were conducted to evaluate safety, tolerability, and analgesic efficacy of single doses of tanezumab, a humanized anti-nerve growth factor monoclonal antibody, in chronic or acute pain.
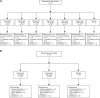
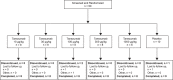
Methods: In the first study (CL001), patients with moderate to severe pain from osteoarthritis (OA) of the knee received a single intravenous infusion of tanezumab (3-1000 μg/kg) or placebo in a dose-escalation (part 1; N = 42) or parallel-arm (part 2; N = 79) study design. The second study (CL002) was a placebo-controlled dose-escalation (tanezumab 10-1000 μg/kg; N = 50) study in patients undergoing bunionectomy surgery.
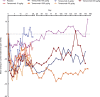
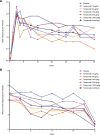
Results: Adverse event rates were generally similar across treatments. Most adverse events were generally mild to moderate in severity and no patients discontinued as a result of adverse events. Adverse events of abnormal peripheral sensation were more common with higher doses of tanezumab (≥100 μg/kg) than with placebo. These were generally mild to moderate in severity. Tanezumab provided up to 12 weeks of effective analgesia for OA knee pain, with statistically significant improvements at doses ≥100 μg/kg (P < 0.05). By contrast, no trend for analgesic activity was found when tanezumab was administered 8 to 16 hours before bunionectomy.
Conclusions: The demonstration of a favorable safety profile and clinical efficacy in OA pain supports clinical development of tanezumab as a potential treatment for chronic pain conditions.
Keywords: Analgesia; Bunionectomy; Nerve growth factor; Osteoarthritis; Safety; Tanezumab.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures


References
-
- Balanescu AR, Feist E, Wolfram G, Davignon I, Smith MD, Brown MT, West CR. Efficacy and safety of tanezumab added on to diclofenac sustained release in patients with knee or hip osteoarthritis: a double-blind, placebo-controlled, parallel-group, multicentre phase III randomised clinical trial. Ann Rheum Dis 2014;73:1665–72. - PubMed
-
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. - PubMed
-
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol 1998;12:43–55.
-
- Bensen WG, Fiechtner JJ, McMillen JI, Zhao WW, Yu SS, Woods EM, Hubbard RC, Isakson PC, Verburg KM, Geis GS. Treatment of osteoarthritis with celecoxib, a cyclooxygenase-2 inhibitor: a randomized controlled trial. Mayo Clin Proc 1999;74:1095–105. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
