Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury
- PMID: 29923765
- PMCID: PMC6230726
- DOI: 10.1152/ajprenal.00072.2018
Physiological functions of ferroportin in the regulation of renal iron recycling and ischemic acute kidney injury
Abstract
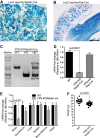
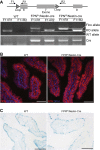
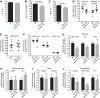
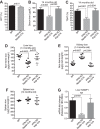
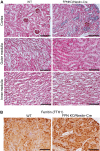
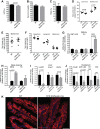
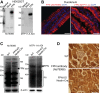
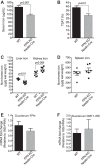
Renal iron recycling preserves filtered iron from urinary excretion. However, it remains debated whether ferroportin (FPN), the only known iron exporter, is functionally involved in renal iron recycling and whether renal iron recycling is required for systemic iron homeostasis. We deleted FPN in whole nephrons by use of a Nestin-Cre and in the distal nephrons and collecting ducts, using a Ksp-Cre, and investigated its impacts on renal iron recycling and systemic iron homeostasis. FPN deletion by Nestin-Cre, but not by Ksp-Cre, caused excess iron retention and increased ferritin heavy chain (FTH1) specifically in the proximal tubules and resulted in the reduction of serum and hepatic iron. The systemic iron redistribution was aggravated, resulting in anemia and the marked downregulation of hepatic hepcidin in elderly FPN knockout (KO)/Nestin-Cre mice. Similarly, in iron-deficient FPN KO/Nestin-Cre mice, the renal iron retention worsened anemia with the activation of the erythropoietin-erythroferrone-hepcidin pathway and the downregulation of hepatic hepcidin. Hence, FPN likely located at the basolateral membrane of the proximal tubules to export iron into the circulation and was required for renal iron recycling and systemic iron homeostasis particularly in elderly and iron-deficient mice. Moreover, FPN deletion in the proximal tubules alleviated ischemic acute kidney injury, possibly by upregulating FTH1 to limit catalytic iron and by priming antioxidant mechanisms, indicating that FPN could be deleterious in the pathophysiology of ischemic acute kidney injury (AKI) and thus may be a potential target for the prevention and mitigation of ischemic AKI.
Keywords: acute kidney injury; ferroportin; iron reabsorption; ischemia/reperfusion.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

