Comparison of Leukosan SkinLink with surgical suture for traumatic laceration repair: A randomized controlled trial
- PMID: 29923977
- PMCID: PMC6023685
- DOI: 10.1097/MD.0000000000010918
Comparison of Leukosan SkinLink with surgical suture for traumatic laceration repair: A randomized controlled trial
Abstract
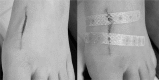
Background: Leukosan SkinLink (LS), which combines non-woven textile strips and tissue adhesive, offers the advantage of atraumatic treatment while effectively shortening the procedure time. We hypothesized that wound closure would be faster with LS than with surgical suture (SS) and the wound infection and dehiscence would be similar.
Methods: A prospective, open label, single-center, randomized controlled trial was performed. Between November 2014 and April 2016, 49 patients with traumatic lacerations who presented to the emergency department were eligible for study inclusion.
Results: The mean wound closure time was significantly lower in the LS group than in the SS group (1.48 ± 0.2 seconds vs 8.8 ± 3.6 minutes, P < .001). After adjusting the wound closure time according to the lacerations length, the time remained significantly lower in the LS group than in the SS group (1.0 ± 0.8 seconds vs 5.03 ± 2.5minutes, P < .001). During follow-up for 14 days, no significant differences in dehiscence and wound infection were observed between the 2 groups.
Conclusion: Wound closure was approximately 4minutes faster with LS and there were no differences in wound infection and dehiscence rates. Thus, the LS could be used as a timesaving suture technique for acute traumatic lacerations in emergency department (ED).
Trial registration: ClinicalTrials.gov NCT02333877.
Conflict of interest statement
All authors declare that they do not have any potential conflicts of interest.
Figures
References
-
- Hollander JE, Singer AJ. Laceration management. Ann Emerg Med 1999;34:356–67. - PubMed
-
- Colovic R. Wound treatment in ancient Greece. Acta Chir Iugosl 2001;48:6–8. - PubMed
-
- Takayama S, Yamamoto T, Tsuchiya C, et al. Comparing Steri-Strip and surgical staple wound closures after primary total knee arthroplasties. Eur J Orthop Surg Traumatol 2017;27:113–8. - PubMed
-
- Singer AJ, Quinn JV, Clark RE, et al. “TraumaSeal Study Group”. Closure of lacerations and incisions with octylcyanoacrylate: a multicenter randomized controlled trial. Surgery 2002;131:270–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous