Unexpected and durable response with regorafenib in a metastatic colorectal cancer patient without KDR mutation: A case report
- PMID: 29924031
- PMCID: PMC6023677
- DOI: 10.1097/MD.0000000000011178
Unexpected and durable response with regorafenib in a metastatic colorectal cancer patient without KDR mutation: A case report
Abstract
Rationale: Regorafenib is an oral multikinase inhibitor and is approved as salvage therapy in the standard treatment of advanced colorectal cancer (CRC). Due to its limited efficacy, toxicity profile, and cost, it is necessary to identify those patients who may have the most benefit from regorafenib. In a previous case report, kinase insert domain receptor (KDR) mutation has been associated with exceptional clinical response (CR) in an elderly patient treated with a low dose of regorafenib; thus, it was hypothesized that it could represent a new predictive marker of drug response.
Patient concerns: A heavily pretreated 67-year-old man with a wide peripancreatic recurrence of colon carcinoma and liver metastases was subjected to treatment with regorafenib.
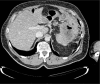
Diagnoses: After 3 months of therapy, a computed tomography scan showed an impressive reduction of disease.
Interventions: Regorafenib was given at full doses (160 mg/die for 21 days, every 4 weeks).
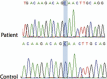
Outcomes: A lasting response without relevant toxicity. No KDR mutation relief was detected. After 13 months from the start of treatment, the patient died after the diagnosis of encephalic metastases.
Lessons: Regorafenib can lead to an unexpected and durable CR with consistent progression-free survival and overall survival benefit even in patients affected by polychemotherapy refractory metastatic CRC. Further studies are needed to establish the benefit of KDR mutation as predictive marker for regorafenib sensitivity for patients with CRC. We include a detailed revision of prognostic and predictive factors of clinical outcome identified in literature to optimize the use of regorafenib in this setting.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures

References
-
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658–67. - PubMed
-
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12. - PubMed
-
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619–29. - PubMed
-
- Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016;16:412. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

