Effects of Cryotherapy on Objective and Subjective Symptoms of Paclitaxel-Induced Neuropathy: Prospective Self-Controlled Trial
- PMID: 29924336
- PMCID: PMC6007752
- DOI: 10.1093/jnci/djx178
Effects of Cryotherapy on Objective and Subjective Symptoms of Paclitaxel-Induced Neuropathy: Prospective Self-Controlled Trial
Abstract
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting and disabling side effect of taxane anticancer agents. We prospectively evaluated the efficacy of cryotherapy for CIPN prevention.
Methods: Breast cancer patients treated weekly with paclitaxel (80 mg/m2 for one hour) wore frozen gloves and socks on the dominant side for 90 minutes, including the entire duration of drug infusion. Symptoms on the treated sides were compared with those on the untreated (nondominant) sides. The primary end point was CIPN incidence assessed by changes in tactile sensitivity from pretreatment baseline in a monofilament test at a cumulative dose of 960 mg/m2. We also assessed thermosensory deficits, subjective symptoms (Patient Neuropathy Questionnaire [PNQ]), manipulative dexterity, and the time to events and hazard ratio by PNQ. All statistical tests were two-sided.
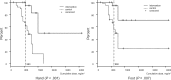
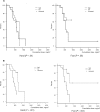
Results: Among the 40 patients, four did not reach the cumulative dose (due to the occurrence of pneumonia, severe fatigue, severe liver dysfunction, and macular edema), leaving 36 patients for analysis. None dropped out due to cold intolerance. The incidence of objective and subjective CIPN signs was clinically and statistically significantly lower on the intervention side than on the control (hand: tactile sensitivity = 27.8% vs 80.6%, odds ratio [OR] = 20.00, 95% confidence interval [CI] = 3.20 to 828.96, P < .001; foot: tacile sensitivity = 25.0% vs 63.9%, OR = infinite, 95% CI = 3.32 to infinite, P < .001; hand: warm sense = 8.8% vs 32.4%, OR = 9.00, 95% CI = 1.25 to 394.48, P = .02; foot: warm sense: 33.4% vs 57.6%, OR = 5.00, 95% CI = 1.07 to 46.93, P = .04; hand: PNQ = 2.8% vs 41.7%, OR = infinite, 95% CI = 3.32 to infinite, P < .001; foot: PNQ = 2.8% vs 36.1%, OR = infinite, 95% CI = 2.78 to infinite, P < .001; hand: hazard ratio [HR] = 0.13, 95% CI = 0.05 to 0.34; foot: HR = 0.13, 95% CI = 0.04 to 0.38, dexterity mean delay = -2.5 seconds, SD = 12.0 seconds, vs + 8.6 seconds, SD = 25.8 seconds, P = .005).
Conclusions: Cryotherapy is useful for preventing both the objective and subjective symptoms of CIPN and resultant dysfunction.
Figures



References
-
- Cavaletti G, Marmiroli P.. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. 2010;6(12):657–666. - PubMed
-
- Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L.. Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer. 2014;22(8):2261–2269. - PubMed
-
- Speck RM, Sammel MD, Farrar JT, et al. . Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J Oncol Pract. 2013;9(5):e234–e240. - PubMed
-
- Hershman DL, Lacchetti C, Dworkin RH, et al. . Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

