Abnormally reduced primary motor cortex output is related to impaired hand function in chronic stroke
- PMID: 29924707
- PMCID: PMC6230804
- DOI: 10.1152/jn.00715.2017
Abnormally reduced primary motor cortex output is related to impaired hand function in chronic stroke
Abstract
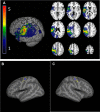
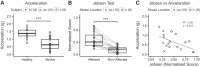
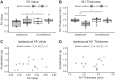
Stroke often involves primary motor cortex (M1) and its corticospinal projections (CST). As hand function is critically dependent on these structures, its recovery is often incomplete. The neuronal substrate supporting affected hand function is not well understood but likely involves reorganized M1 and CST of the lesioned hemisphere (M1IL and CSTIL). We hypothesized that affected hand function in chronic stroke is related to structural and functional reorganization of M1IL and CSTIL. We tested 18 patients with chronic ischemic stroke involving M1 or CST. Their hand function was compared with 18 age-matched healthy subjects. M1IL thickness and CSTIL fractional anisotropy (FA) were determined with MRI and compared with measures of the other hemisphere. Transcranial magnetic stimulation (TMS) was applied to M1IL to determine its input-output function [stimulus response curve (SRC)]. The plateau of the SRC (MEPmax), inflection point, and slope parameters of the curve were extracted. Results were compared with measures in 12 age-matched healthy controls. MEPmax of M1IL was significantly smaller ( P = 0.02) in the patients, indicating reduced CSTIL motor output, and was correlated with impaired hand function ( P = 0.02). M1IL thickness ( P < 0.01) and CSTIL-FA ( P < 0.01) were reduced but did not correlate with hand function. The results indicate that employed M1IL or CSTIL structural measures do not explain the extent of impairment in hand function once M1 and CST are sufficiently functional for TMS to evoke a motor potential. Instead, impairment of hand function is best explained by the abnormally low output from M1IL. NEW & NOTEWORTHY Hand function often remains impaired after stroke. While the critical role of the primary motor cortex (M1) and its corticospinal output (CST) for hand function has been described in the nonhuman primate stroke model, their structure and function have not been systematically evaluated for patients after stroke. We report that in chronic stroke patients with injury to M1 and/or CST an abnormally reduced M1 output is related to impaired hand function.
Keywords: human; rehabilitation; stroke.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

