Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss
- PMID: 29924955
- PMCID: PMC6117111
- DOI: 10.1056/NEJMoa1801109
Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss
Abstract
Background: Cisplatin chemotherapy and surgery are effective treatments for children with standard-risk hepatoblastoma but may cause considerable and irreversible hearing loss. This trial compared cisplatin with cisplatin plus delayed administration of sodium thiosulfate, aiming to reduce the incidence and severity of cisplatin-related ototoxic effects without jeopardizing overall and event-free survival.
Methods: We randomly assigned children older than 1 month and younger than 18 years of age who had standard-risk hepatoblastoma (≤3 involved liver sectors, no metastatic disease, and an alpha-fetoprotein level of >100 ng per milliliter) to receive cisplatin alone (at a dose of 80 mg per square meter of body-surface area, administered over a period of 6 hours) or cisplatin plus sodium thiosulfate (at a dose of 20 g per square meter, administered intravenously over a 15-minute period, 6 hours after the discontinuation of cisplatin) for four preoperative and two postoperative courses. The primary end point was the absolute hearing threshold, as measured by pure-tone audiometry, at a minimum age of 3.5 years. Hearing loss was assessed according to the Brock grade (on a scale from 0 to 4, with higher grades indicating greater hearing loss). The main secondary end points were overall survival and event-free survival at 3 years.
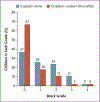
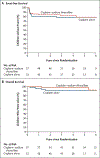
Results: A total of 109 children were randomly assigned to receive cisplatin plus sodium thiosulfate (57 children) or cisplatin alone (52) and could be evaluated. Sodium thiosulfate was associated with few high-grade toxic effects. The absolute hearing threshold was assessed in 101 children. Hearing loss of grade 1 or higher occurred in 18 of 55 children (33%) in the cisplatin-sodium thiosulfate group, as compared with 29 of 46 (63%) in the cisplatin-alone group, indicating a 48% lower incidence of hearing loss in the cisplatin-sodium thiosulfate group (relative risk, 0.52; 95% confidence interval [CI], 0.33 to 0.81; P=0.002). At a median of 52 months of follow-up, the 3-year rates of event-free survival were 82% (95% CI, 69 to 90) in the cisplatin-sodium thiosulfate group and 79% (95% CI, 65 to 88) in the cisplatin-alone group, and the 3-year rates of overall survival were 98% (95% CI, 88 to 100) and 92% (95% CI, 81 to 97), respectively.
Conclusions: The addition of sodium thiosulfate, administered 6 hours after cisplatin chemotherapy, resulted in a lower incidence of cisplatin-induced hearing loss among children with standard-risk hepatoblastoma, without jeopardizing overall or event-free survival. (Funded by Cancer Research UK and others; SIOPEL 6 ClinicalTrials.gov number, NCT00652132 ; EudraCT number, 2007-002402-21 .).
Figures
Comment in
-
Sodium thiosulfate halves the risk of cisplatin-induced hearing loss.Nat Rev Clin Oncol. 2018 Sep;15(9):533. doi: 10.1038/s41571-018-0067-2. Nat Rev Clin Oncol. 2018. PMID: 29980744 No abstract available.
-
Sodium Thiosulfate and Cisplatin-Induced Hearing Loss.N Engl J Med. 2018 Sep 20;379(12):1180-1. doi: 10.1056/NEJMc1809501. N Engl J Med. 2018. PMID: 30260148 Free PMC article. No abstract available.
References
-
- Brown J, Perilongo G, Shafford E, et al. Pretreatment prognostic factors for children with hepatoblastoma — results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer 2000;36:1418–25. - PubMed
-
- Maibach R, Roebuck D, Brugieres L, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer 2012;48:1543–9. - PubMed
-
- Perilongo G, Maibach R, Shafford E, et al. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med 2009;361:1662–70. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
