Replication Fork Reversal during DNA Interstrand Crosslink Repair Requires CMG Unloading
- PMID: 29924986
- PMCID: PMC6086610
- DOI: 10.1016/j.celrep.2018.05.061
Replication Fork Reversal during DNA Interstrand Crosslink Repair Requires CMG Unloading
Abstract
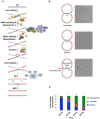
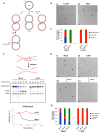
DNA interstrand crosslinks (ICLs) are extremely cytotoxic, but the mechanism of their repair remains incompletely understood. Using Xenopus egg extracts, we previously showed that repair of a cisplatin ICL is triggered when two replication forks converge on the lesion. After CDC45/MCM2-7/GINS (CMG) ubiquitylation and unloading by the p97 segregase, FANCI-FANCD2 promotes DNA incisions by XPF-ERCC1, leading to ICL unhooking. Here, we report that, during this cell-free ICL repair reaction, one of the two converged forks undergoes reversal. Fork reversal fails when CMG unloading is inhibited, but it does not require FANCI-FANCD2. After one fork has undergone reversal, the opposing fork that still abuts the ICL undergoes incisions. Our data show that replication fork reversal at an ICL requires replisome disassembly. We present a revised model of ICL repair that involves a reversed fork intermediate.
Keywords: CMG; DNA interstrand crosslink repair; DNA replication; Fanconi anemia; ICL; XPF; replication fork reversal.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

