Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells
- PMID: 29925006
- PMCID: PMC6063084
- DOI: 10.1016/j.celrep.2018.05.068
Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells
Abstract
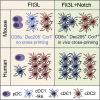
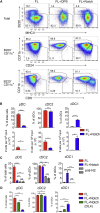
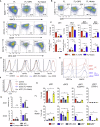
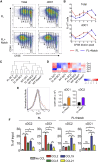
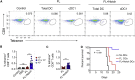
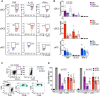
The IRF8-dependent subset of classical dendritic cells (cDCs), termed cDC1, is important for cross-priming cytotoxic T cell responses against pathogens and tumors. Culture of hematopoietic progenitors with DC growth factor FLT3 ligand (FLT3L) yields very few cDC1s (in humans) or only immature "cDC1-like" cells (in the mouse). We report that OP9 stromal cells expressing the Notch ligand Delta-like 1 (OP9-DL1) optimize FLT3L-driven development of cDC1s from murine immortalized progenitors and primary bone marrow cells. Co-culture with OP9-DL1 induced IRF8-dependent cDC1s with a phenotype (CD103+ Dec205+ CD8α+) and expression profile resembling primary splenic cDC1s. OP9-DL1-induced cDC1s showed preferential migration toward CCR7 ligands in vitro and superior T cell cross-priming and antitumor vaccination in vivo. Co-culture with OP9-DL1 also greatly increased the yield of IRF8-dependent CD141+ cDC1s from human bone marrow progenitors cultured with FLT3L. Thus, Notch signaling optimizes cDC generation in vitro and yields authentic cDC1s for functional studies and translational applications.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
'NOTCHing up' the In Vitro Production of Dendritic Cells.Trends Immunol. 2018 Oct;39(10):765-767. doi: 10.1016/j.it.2018.08.002. Epub 2018 Aug 24. Trends Immunol. 2018. PMID: 30150090
References
-
- Aliberti J., Schulz O., Pennington D.J., Tsujimura H., Reis e Sousa C., Ozato K., Sher A. Essential role for ICSBP in the in vivo development of murine CD8alpha + dendritic cells. Blood. 2003;101:305–310. - PubMed
-
- Balan S., Ollion V., Colletti N., Chelbi R., Montanana-Sanchis F., Liu H., Vu Manh T.P., Sanchez C., Savoret J., Perrot I. Human XCR1+ dendritic cells derived in vitro from CD34+ progenitors closely resemble blood dendritic cells, including their adjuvant responsiveness, contrary to monocyte-derived dendritic cells. J. Immunol. 2014;193:1622–1635. - PMC - PubMed
-
- Bigley V., Maisuria S., Cytlak U., Jardine L., Care M.A., Green K., Gunawan M., Milne P., Dickinson R., Wiscombe S. Biallelic interferon regulatory factor 8 mutation: A complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J. Allergy Clin. Immunol. 2017 S0091-6749(17)31736-0. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

